L'augmentation de l'incidence et de l'épidémiologie de l'hypophosphatasie (HPP) est un facteur clé de la croissance du marché du traitement de cette maladie rare. Cette incidence croissante incite de plus en plus de personnes à rechercher des options thérapeutiques, ce qui incite les laboratoires pharmaceutiques à investir dans la recherche et le développement de nouvelles thérapies. Le marché des traitements contre l'HPP connaît une croissance rapide, les professionnels de santé comme les patients reconnaissant la nécessité d'interventions efficaces pour améliorer la qualité de vie et gérer les symptômes associés à cette maladie complexe.
Accéder au rapport complet sur https://www.databridgemarketresearch.com/reports/global-hypophosphatasia-treatment-market
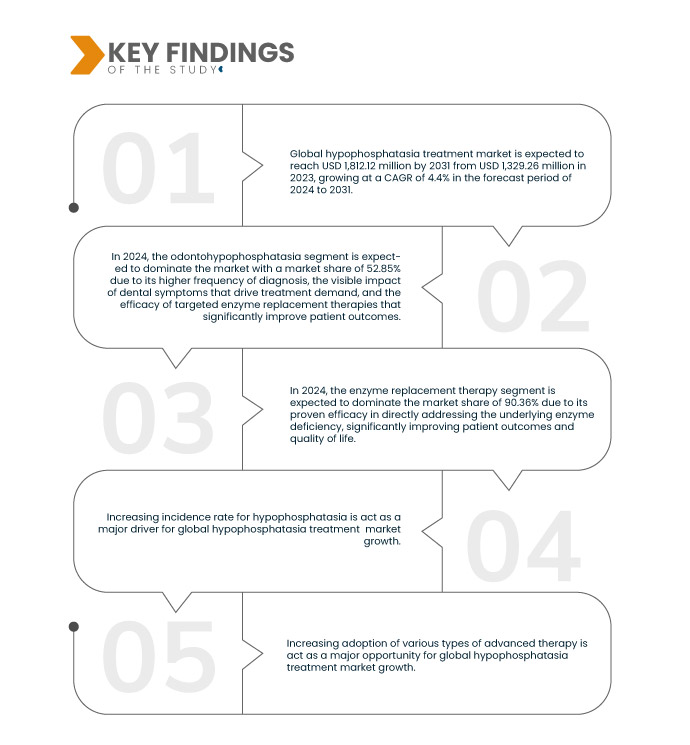
Data Bridge Market Research analyse que le marché mondial du traitement de l'hypophosphatasie devrait atteindre 1 812,12 millions USD d'ici 2031, contre 1 329,26 millions USD en 2023, avec un TCAC de 4,4 % au cours de la période de prévision de 2024 à 2031.
Principales conclusions de l'étude
Politiques de remboursement favorables
Des politiques de remboursement favorables jouent un rôle crucial dans la disponibilité et l'accessibilité des traitements contre l'hypophosphatasie (HPP). Lorsque les systèmes de santé et les assurances proposent un remboursement solide des traitements contre l'HPP, les obstacles financiers pour les patients et les professionnels de santé sont réduits, facilitant ainsi un accès plus large à ces traitements spécialisés.
Dans les pays où les politiques de remboursement sont favorables, les laboratoires pharmaceutiques sont davantage incités à développer et commercialiser des traitements contre l'HPP. Cela peut favoriser un marché plus concurrentiel, offrant de multiples options thérapeutiques, favorisant l'innovation et améliorant les résultats thérapeutiques pour les patients. De plus, des politiques de remboursement favorables encouragent les professionnels de santé à prescrire ces traitements en toute confiance, sachant que les patients peuvent les financer sans fardeau financier excessif.
Portée du rapport et segmentation du marché
Rapport métrique
|
Détails
|
Période de prévision
|
2024 à 2031
|
Année de base
|
2023
|
Année historique
|
2022 (personnalisable de 2016 à 2021)
|
Unités quantitatives
|
Chiffre d'affaires en millions USD
|
Segments couverts
|
Types (odontohypophosphatasie, pseudohypophosphatasie et autres), type de traitement ( traitement enzymatique substitutif et traitement de soutien), voie d'administration (injectable et orale), utilisateur final (hôpitaux, cliniques spécialisées et autres), canal de distribution (pharmacie hospitalière, pharmacie de détail et pharmacie en ligne)
|
Acteurs du marché couverts
|
AstraZeneca (Royaume-Uni), Pfizer Inc. (États-Unis), Abbott (États-Unis), Novartis AG (Suisse), Be Biopharma (États-Unis), PuREC (Japon), Rallybio (États-Unis), Rampart Bioscience, Inc. (États-Unis), AM-Pharma BV (Pays-Bas) et Roivant Sciences Ltd. (États-Unis), entre autres
|
Points de données couverts dans le rapport
|
En plus des informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire.
|
Analyse des segments
Le marché mondial du traitement de l’hypophosphatasie est segmenté en cinq segments notables en fonction des types, du type de thérapie, de la voie d’administration, de l’utilisateur final et du canal de distribution.
- Sur la base des types, le marché mondial du traitement de l'hypophosphatasie est segmenté en odontohypophosphatasie, pseudohypophosphatasie et autres
En 2024, le segment de l'odontohypophosphatasie devrait dominer le marché mondial du traitement de l'hypophosphatasie
En 2024, le segment de l'odontohypophosphatasie devrait dominer le marché avec une part de marché de 52,85 % en raison de sa fréquence de diagnostic plus élevée, de l'impact visible des symptômes dentaires qui stimulent la demande de traitement et de l'efficacité des thérapies de remplacement enzymatique ciblées qui améliorent considérablement les résultats des patients.
- Sur la base du type de thérapie, le marché mondial du traitement de l'hypophosphatasie est segmenté en thérapie de remplacement enzymatique et thérapie de soutien.
En 2024, le segment de la thérapie de remplacement enzymatique devrait dominer le marché mondial du traitement de l'hypophosphatasie
En 2024, le segment de la thérapie de remplacement enzymatique devrait dominer la part de marché de 90,36 % en raison de son efficacité prouvée pour traiter directement la déficience enzymatique sous-jacente, améliorant considérablement les résultats des patients et leur qualité de vie.
- Selon la voie d'administration, le marché mondial du traitement de l'hypophosphatasie est segmenté en deux catégories : injectables et orales. En 2024, le segment injectable devrait dominer le marché avec une part de marché de 86,09 %.
- En fonction de l'utilisateur final, le marché mondial du traitement de l'hypophosphatasie est segmenté entre hôpitaux, cliniques spécialisées et autres. En 2024, le segment hospitalier devrait dominer le marché avec une part de marché de 69,98 %.
- En fonction des canaux de distribution, le marché mondial du traitement de l'hypophosphatasie est segmenté en pharmacies hospitalières, pharmacies de détail et pharmacies en ligne. En 2024, le segment des pharmacies hospitalières devrait dominer le marché avec une part de marché de 59,04 %.
Acteurs majeurs
Data Bridge Market Research reconnaît les sociétés suivantes comme les principaux acteurs du marché mondial du traitement de l'hypophosphatasie : AstraZeneca (Royaume-Uni), Pfizer Inc. (États-Unis), Abbott (États-Unis), Novartis AG (Suisse).
Évolution du marché
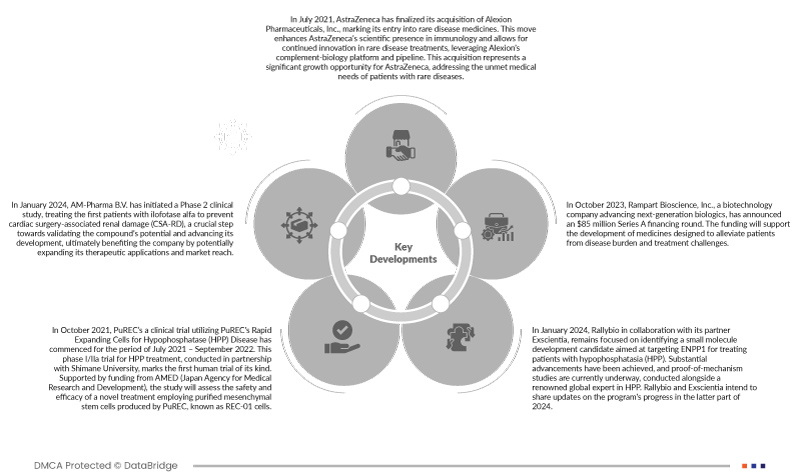
- En juillet 2021, AstraZeneca a finalisé l'acquisition d'Alexion Pharmaceuticals, Inc., marquant ainsi son entrée sur le marché des médicaments contre les maladies rares. Cette opération renforce la présence scientifique d'AstraZeneca en immunologie et lui permet de poursuivre l'innovation dans le traitement des maladies rares, en s'appuyant sur la plateforme et le portefeuille de produits d'Alexion en biologie du complément. Cette acquisition représente une opportunité de croissance significative pour AstraZeneca, répondant aux besoins médicaux non satisfaits des patients atteints de maladies rares.
- En janvier 2024, AM-Pharma BV a lancé une étude clinique de phase 2, traitant les premiers patients avec de l'ilofotase alfa pour prévenir les lésions rénales associées à la chirurgie cardiaque (CSA-RD), une étape cruciale vers la validation du potentiel du composé et l'avancement de son développement, bénéficiant finalement à l'entreprise en élargissant potentiellement ses applications thérapeutiques et sa portée commerciale.
- En octobre 2023, AM-Pharma BV a annoncé des résultats cliniques positifs de phase 1b pour l'ilofotase alfa, un traitement enzymatique substitutif potentiel chez les patients adultes atteints d'hypophosphatasie (HPP). Cette étape importante démontre l'efficacité et l'innocuité du composé, élargissant potentiellement son marché et ses applications thérapeutiques, et favorisant ainsi la croissance et le développement de l'entreprise.
- En mai, à CAMBRIDGE, dans le Massachusetts, Be Biopharma, Inc. (« Be Bio »), une société pionnière dans la découverte et le développement de médicaments à base de cellules B modifiées (BCM), a présenté les résultats d'une nouvelle recherche préclinique démontrant la production d'ALP active par un BCM, ce qui met en évidence les BCM comme un traitement potentiel de l'hypophosphatasie (HPP).
- En octobre 2021, un essai clinique utilisant les cellules à expansion rapide de PuREC pour le traitement de l'hypophosphatase (HPP) a débuté pour la période de juillet 2021 à septembre 2022. Cet essai de phase I/IIa pour le traitement de l'HPP, mené en partenariat avec l'Université de Shimane, est le premier essai de ce type chez l'homme. Financée par l'AMED (Agence japonaise pour la recherche et le développement médicaux), cette étude évaluera l'innocuité et l'efficacité d'un nouveau traitement utilisant des cellules souches mésenchymateuses purifiées produites par PuREC, appelées cellules REC-01.
- En janvier 2024, Rallybio, en collaboration avec son partenaire Exscientia, poursuit ses efforts pour identifier une petite molécule candidate ciblant ENPP1 pour le traitement des patients atteints d'hypophosphatasie (HPP). Des avancées substantielles ont été réalisées et des études de preuve de mécanisme sont actuellement en cours, menées en collaboration avec un expert mondial reconnu en HPP. Rallybio et Exscientia prévoient de partager l'état d'avancement du programme fin 2024.
- En octobre 2023, Rampart Bioscience, Inc., une société de biotechnologie spécialisée dans les produits biologiques de nouvelle génération, a annoncé un tour de financement de série A de 85 millions de dollars. Ce financement soutiendra le développement de médicaments destinés à alléger le fardeau de la maladie et à soulager les difficultés thérapeutiques des patients.
Analyse régionale
Géographiquement, les pays couverts dans le rapport sur le marché mondial du traitement de l'hypophosphatasie sont les États-Unis, le Canada, le Mexique, l'Allemagne, le Royaume-Uni, la France, l'Italie, les Pays-Bas, l'Espagne, la Russie, la Suisse, la Turquie, la Belgique, le reste de l'Europe, la Chine, le Japon, l'Inde, la Corée du Sud, l'Australie, Singapour, la Thaïlande, l'Indonésie, la Malaisie, les Philippines, le reste de l'Asie-Pacifique, le Brésil, l'Argentine, le reste de l'Amérique du Sud, l'Arabie saoudite, les Émirats arabes unis, Israël, l'Afrique du Sud, l'Égypte et le reste du Moyen-Orient et de l'Afrique.
Selon l'analyse de Data Bridge Market Research :
L'Amérique du Nord est la région dominante sur le marché mondial du traitement de l'hypophosphatasie
L'Amérique du Nord devrait dominer le marché grâce à son adoption précoce des thérapies avancées et à la solidité de ses infrastructures de santé. L'Amérique du Nord continuera de dominer le marché en termes de parts de marché et de chiffre d'affaires, et sa domination se poursuivra durant la période de prévision. Ceci est dû à l'adoption croissante des technologies de pointe dans cette région.
L'Asie-Pacifique est considérée comme la région connaissant la croissance la plus rapide sur le marché mondial du traitement de l'hypophosphatasie.
La région Asie-Pacifique devrait connaître une croissance au cours de la période de prévision grâce à une sensibilisation accrue, à l'amélioration des infrastructures de santé et à l'augmentation de la population de patients. De plus, la hausse des dépenses de santé et du revenu par habitant devrait stimuler la croissance du marché dans cette région.
Pour plus d'informations sur le marché mondial du traitement de l'hypophosphatasie, cliquez ici : https://www.databridgemarketresearch.com/reports/global-hypophosphatasia-treatment-market