Согласно информационному бюллетеню IQVIA, в июле 2022 года ее система запросов данных (DQS) использовала управление основными данными в надежном репозитории данных с возможностью поиска, что позволяет спонсорам и CRO находить наиболее подходящих исследователей и площадки для своих клинических испытаний, а также лучше поддерживать планирование исследований.
Полный отчет доступен по адресу https://www.databridgemarketresearch.com/reports/global-eclinical-solutions-market
Более того, по данным Национального центра статистики науки и техники (NCSES), в 2020 году США потратили 538 млрд долларов США на исследования и разработки, увеличив эффективность на 9,1% по сравнению с расходами в 2019 году.
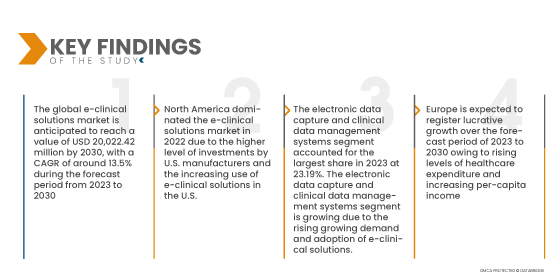
По данным исследования рынка Data Bridge, ожидается, что глобальный рынок электронных клинических решений будет расти среднегодовыми темпами в 13,5% в прогнозируемый период с 2023 по 2030 год и достигнет 20 022,42 млн долларов США к 2030 году. Прогнозируется, что электронные системы сбора данных и управления клиническими данными будут способствовать росту рынка, поскольку они широко используются в электронных клинических решениях для сбора данных клинических испытаний и анализа медицинских данных в электронном виде.
Основные выводы исследования
Растущее использование электронных клинических решений в клинических испытаниях
Клиническое испытание — это исследовательская процедура, используемая для изучения разработки лекарственных средств с целью оценки безопасности и эффективности молекулы. Клиническое испытание — это обширный и сложный процесс, который повысил спрос на инновационное и автоматизированное электронное решение для упрощения и сокращения продолжительности клинических испытаний. Исследователи и производители разработали автоматизированные электронные методы для предоставления оценки в системах управления клиническими испытаниями.
Решения e-clinical включают электронные медицинские карты, электронные формы согласия, интеграцию электронных технологий, электронный сбор данных и системы управления клиническими данными. Решения e-clinical помогают исследователям в сквозных процессах клинических исследований, предоставляя решения посредством надлежащего управления длительными процессами клинических исследований. Они помогают организациям клинических исследований с управлением нормативными документами, совместной работой в команде и управлением цепочками поставок, управлением производительностью сайта и отчетностью, что увеличивает спрос на решения e-Clinical на рынке.
Решения e-clinical помогли врачам и исследователям сократить стоимость и продолжительность клинических испытаний, а также собрать данные. Таким образом, снизить вероятность потери данных и увеличить количество клинических испытаний, что увеличивает использование решений e-clinical в клинических испытаниях.
Область отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2023-2030
|
Базовый год
|
2022
|
Исторические годы
|
2021 (Можно настроить на 2015-2020)
|
Количественные единицы
|
Доход в миллионах, цены в долларах США
|
Охваченные сегменты
|
По продукту (электронные системы сбора данных и управления данными клинических испытаний, системы управления клиническими испытаниями, платформы клинической аналитики, медицинские записи для координации ухода (CCMR), рандомизация и управление поставками для испытаний, платформы интеграции клинических данных, электронные решения для оценки клинических результатов, решения по безопасности, электронные системы основных файлов испытаний, решения по управлению нормативной информацией и другие), способ доставки (решения, размещенные в Интернете (по запросу), лицензированные корпоративные (локальные) решения и облачные (SAAS) решения), фаза клинических испытаний (фаза I, фаза II, фаза III и фаза IV), размер организации (малая, средняя и большая), пользовательское устройство (настольный компьютер, планшет, карманное устройство PDA, смартфон и другие), конечный пользователь (фармацевтические и биофармацевтические компании, организации по контрактным исследованиям, компании по оказанию консалтинговых услуг, производители медицинского оборудования, больницы и научно-исследовательские институты)
|
Страны, охваченные
|
США, Канада, Мексика, Германия, Франция, Великобритания, Италия, Россия, Испания, Нидерланды, Швейцария, Бельгия, Турция, Остальная Европа, Китай, Япония, Индия, Австралия, Южная Корея, Сингапур, Малайзия, Таиланд, Индонезия, Филиппины, Остальная часть Азиатско-Тихоокеанского региона, Южная Африка, Саудовская Аравия, ОАЭ, Израиль, Египет, Остальной Ближний Восток и Африка, Бразилия, Аргентина и Остальная часть Южной Америки
|
Охваченные участники рынка
|
Oracle (США), Signant Health (США), MaxisIT (США), Paraxel International Corporation (США), Dassault Systemes (Франция), Clario (США), Mednet (США), OpenClinica, LLC (США), 4G Clinical (США), Veeva Systems (США), Saama Technologies, LLC (США), Anju (США), Castor, Medrio, Inc. (США), ArisGlobal (США), Merative (США), Advarra (США), eClinical Solutions, LLC (США), Y-Prime LLC (США), RealTime Software Solutions LLC (США), Datatrak Int. (США), IQVIA Inc. (США), Research Manager (Нидерланды) и Quretec (Эстония) и другие
|
Данные, отраженные в отчете
|
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя глубокий экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента:
Рынок электронных клинических решений сегментирован по продукту, способу доставки, фазе клинических испытаний, размеру организации, пользовательскому устройству и конечному пользователю.
- На основе продукта глобальный рынок электронных клинических решений сегментируется на электронные системы сбора данных и управления данными клинических испытаний, системы управления клиническими испытаниями , платформы клинической аналитики, медицинские записи для координации лечения (CCMR), рандомизацию и управление поставками для испытаний, платформы интеграции клинических данных, электронные решения для оценки клинических результатов, решения по безопасности, электронные системы основных файлов испытаний, решения по управлению нормативной информацией и другие.
Ожидается, что в 2022 году сегмент систем электронного сбора данных и управления данными клинических испытаний станет доминирующим на мировом рынке электронных клинических решений.
В 2022 году сегмент систем электронного сбора данных и управления данными клинических испытаний этого рынка будет доминировать на мировом рынке электронных клинических решений из-за растущей осведомленности о преимуществах услуг EDC по сравнению с бумажным управлением клинической информацией. Ожидается, что сегмент систем электронного сбора данных и управления данными клинических испытаний достигнет самого высокого среднегодового темпа роста в 17,3% в прогнозируемый период с 2023 по 2030 год.
- На основе способа доставки рынок сегментируется на веб-хостинговые (по требованию) решения, лицензированные корпоративные (локальные) решения и облачные (SAAS) решения . Ожидается, что в 2023 году сегмент веб-хостинговых (по требованию) решений будет доминировать на мировом рынке электронных клинических решений с долей рынка 39,72% благодаря достижениям и инновациям в подходах электронных клинических решений.
- На основе фазы клинических испытаний рынок сегментируется на фазу I, фазу II, фазу III и фазу IV. Ожидается, что в 2023 году сегмент фазы III будет доминировать на мировом рынке электронных клинических решений с долей рынка 38,28% из-за растущих инвестиций правительства и фармацевтических компаний в клинические испытания и разработку лекарств.
- На основе размера организации рынок сегментируется на малый и средний, и большой. Ожидается, что в 2023 году средний и малый сегмент будет доминировать на мировом рынке электронных клинических решений с долей рынка 62,61% из-за растущих стратегических инициатив различных игроков рынка
- На основе пользовательского устройства рынок сегментируется на настольные компьютеры, планшеты, карманные устройства PDA, смартфоны и др. Ожидается, что в 2023 году сегмент настольных компьютеров будет доминировать на мировом рынке электронных клинических решений с долей рынка 49,57% из-за растущего использования электронных гаджетов среди населения.
- На основе конечного пользователя рынок сегментирован на фармацевтические и биофармацевтические компании, контрактные исследовательские организации, консалтинговые компании, производителей медицинского оборудования, больницы и академические научно-исследовательские институты. Ожидается, что в 2023 году сегмент контрактных исследовательских организаций будет доминировать на мировом рынке электронных клинических решений с долей рынка 40,32% из-за роста числа пациентов с различными расстройствами и роста исследовательской деятельности
Основные игроки
Data Bridge Market Research признает следующие компании основными игроками на мировом рынке электронных клинических решений: Oracle (США), Signant Health (США), MaxisIT (США), Paraxel International Corporation (США), Dassault Systemes (Франция), Clario (США), Mednet (США), OpenClinica, LLC (США), 4G Clinical (США), Veeva Systems (США), Saama Technologies, LLC (США), Anju (США), Castor, Medrio, Inc. (США), ArisGlobal (США), Merative (США), Advarra (США), eClinical Solutions, LLC (США), Y-Prime LLC (США), RealTime Software Solutions LLC (США), Datatrak Int. (США), IQVIA Inc. (США), Research Manager (Нидерланды) и Quretec (Эстония) и другие.
Развитие рынка
- В апреле 2023 года Oracle, ведущий поставщик операционных и клинических технологий для больниц и систем здравоохранения по всему миру, Oracle, Deloitte сегодня объявила о расширении своей операционной системы для отрасли здравоохранения, включив в нее услуги по внедрению, эксплуатации и консультированию для Oracle. Растущий портфель продуктов Deloitte для медицинских технологий, Deloitte Health—Oracle Accelerated, создан для того, чтобы идти в ногу с трансформацией отрасли в обширную экосистему и платформу, управляемую пациентами, а не больницами, поставщиками или страховыми компаниями. Deloitte, давний участник Oracle PartnerNetwork (OPN), сотрудничает с Oracle Health, чтобы предоставить клиентам систему предоставления медицинских услуг, которая готова к будущему
- В апреле 2023 года дочерняя компания Medidata компании Dassault Systèmes объявила, что Lambda Therapeutics внедряет облачные клинические продукты Medidata, Rave EDC, Rave RTSM и Rave Imaging, согласно заявлению дочерней компании Dassault Systèmes Medidata. Автоматизация и оптимизация операций по управлению данными и безопасная доставка данных более высокого качества для более быстрого понимания, это еще больше повысит производительность клинических испытаний. Это помогло бизнесу в продвижении своих предложений по всему миру
- В марте 2023 года Clario запустила облачный инструмент Image Viewer, который помогает спонсорам и CRO просматривать изображения своих клинических испытаний. Ранее нескольким организациям приходилось участвовать в процедуре передачи изображений, чтобы увидеть фотографии для клинического испытания. Это усложняло и без того рискованный процесс и увеличивало вероятность задержек и ошибок. Это помогло бизнесу расширить предложение услуг
- В июле 2022 года, согласно информационному бюллетеню IQVIA, ее система запросов данных (DQS) использовала управление основными данными в надежном репозитории данных с возможностью поиска, что позволяет спонсорам и CRO находить наиболее подходящих исследователей и площадки для своих клинических испытаний и лучше поддерживать планирование исследований.
- В феврале 2022 года компания Advarra, лидер рынка решений для регулирующих проверок, консалтинговых услуг по качеству исследований и соблюдению нормативных требований, а также технологических решений для клинических исследований, объявила о запуске нового курса обучения проведению клинических исследований, направленного на оптимизацию активации сайтов и снижение нагрузки на обучение на сайтах.
- В декабре 2021 года компания Advarra запустила облачную платформу нового поколения для клинических исследований, которая может улучшить доставку приложений для автоматизации, оптимизации, подключения и ускорения процессов на протяжении всего жизненного цикла клинических исследований.
Региональный анализ
Географически в отчете о рынке передовых средств для лечения ран представлены следующие страны: США, Канада, Мексика, Германия, Франция, Великобритания, Италия, Россия, Испания, Нидерланды, Швейцария, Бельгия, Турция, остальные страны Европы, Китай, Япония, Индия, Австралия, Южная Корея, Сингапур, Малайзия, Таиланд, Индонезия, Филиппины, остальные страны Азиатско-Тихоокеанского региона, Южная Африка, Саудовская Аравия, ОАЭ, Израиль, Египет, остальные страны Ближнего Востока и Африки, Бразилия, Аргентина и остальные страны Южной Америки.
Согласно анализу Data Bridge Market Research:
Северная Америка будет доминирующим регионом на мировом рынке электронных клинических решений в прогнозируемый период 2023–2030 гг.
В 2022 году Северная Америка доминировала на мировом рынке электронных клинических решений благодаря более высокому уровню инвестиций со стороны производителей США и растущему принятию электронных клинических решений. Северная Америка продолжит доминировать на рынке электронных клинических решений с точки зрения доли рынка и доходов рынка и продолжит процветать в течение прогнозируемого периода. Это связано с растущим принятием передовых технологий и запуском новых продуктов электронных клинических решений в этом регионе. Кроме того, ожидается увеличение числа клинических исследований по разработке и открытию лекарственных препаратов, и это будет стимулировать спрос на системы EDC в этом регионе.
По оценкам, Северная Америка станет самым быстрорастущим регионом на рынке электронных клинических решений в прогнозируемый период с 2023 по 2030 год.
Ожидается, что Северная Америка будет расти в течение прогнозируемого периода из-за быстрого развития медицинских учреждений в развивающихся экономиках в этом регионе. В дополнение к этому, ожидается, что растущие уровни расходов на здравоохранение и увеличение дохода на душу населения будут способствовать темпам роста рынка в этом регионе.
Более подробную информацию об отчете о рынке электронных клинических решений можно получить здесь - https://www.databridgemarketresearch.com/reports/global-eclinical-solutions-market