L'exome entier est une technique génomique permettant de séquencer toutes les régions codant pour les protéines des gènes d'un génome. Le séquençage complet de l’exome (WES) est disponible pour les patients qui recherchent un diagnostic unificateur pour plusieurs pathologies. Processus de laboratoire utilisé pour déterminer la séquence nucléotidique principalement des régions exoniques (ou codantes pour les protéines) du génome d'un individu et des séquences associées, représentant environ 1 % de la séquence complète d'ADN, également appelée WES. Le séquençage de l’exome entier est une méthode de séquençage de l’exome entier (WES) largement utilisée qui implique le séquençage des régions codant pour les protéines du génome. L’exome humain représente moins de 2 % du génome, mais contient environ 85 % des variantes connues liées à la maladie1, ce qui fait de cette méthode une alternative rentable au séquençage du génome entier.
Accéder au rapport complet @https://www.databridgemarketresearch.com/reports/north-america-whole-exome-sequencing-market
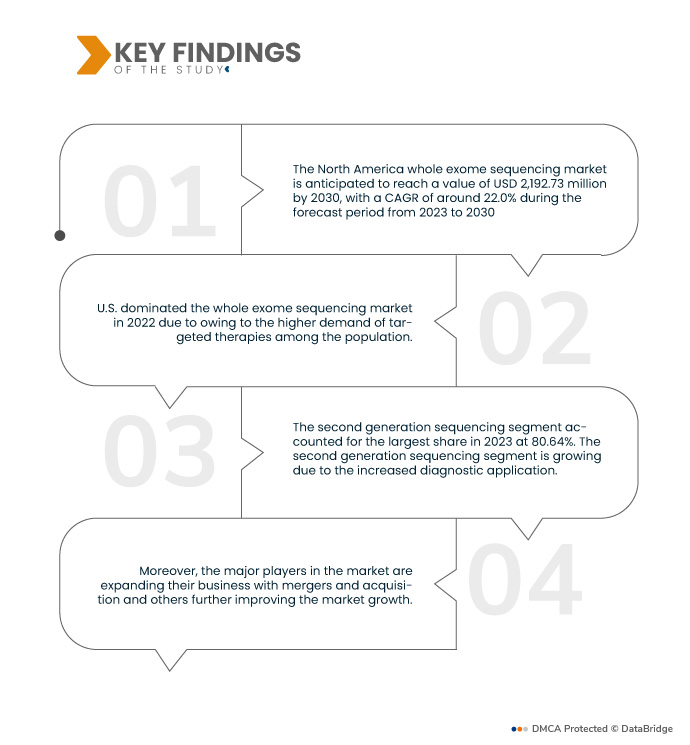
Data Bridge Market Research analyse que le Marché du séquençage de l’exome entier (WES) devrait croître à un TCAC de 22,0 % au cours de la période de prévision de 2023 à 2030 et devrait atteindre 2 192,73 millions de dollars d’ici 2030. Le séquençage de deuxième génération devrait propulser la croissance du marché car il est largement utilisé dans les applications de diagnostic. .
Principales conclusions de l'étude
Utilisation croissante de méthodes de séquençage ciblées
Alors que la pharmacologie axée sur la génomique continue de jouer un rôle plus important dans le traitement de diverses maladies chroniques, en particulier le cancer, le séquençage de nouvelle génération (NGS) évolue pour devenir un outil puissant permettant de fournir une analyse plus approfondie et plus précise des fondements moléculaires des tumeurs individuelles et récepteurs spécifiques.
NGS offre des avantages en termes de précision, de sensibilité et de rapidité par rapport aux méthodes traditionnelles susceptibles d’avoir un impact significatif sur le domaine de l’oncologie. Étant donné que NGS peut évaluer plusieurs gènes en un seul test, le besoin de commander plusieurs tests pour identifier la mutation causale est éliminé.
Portée du rapport et segmentation du marché
|
Mesure du rapport
|
Détails
|
|
Période de prévision
|
2023 à 2030
|
|
Année de référence
|
2022
|
|
Années historiques
|
2021 (personnalisable jusqu’en 2015 – 2020)
|
|
Unités quantitatives
|
Chiffre d'affaires en millions USD
|
|
Segments couverts
|
Composant (séquençage de l'exome entier, séquençage de deuxième génération et Séquençage de troisième génération), produits et services (systèmes, kits et services), applications (diagnostic, découverte et développement de médicaments, médecine personnalisée, agriculture et recherche animale, et autres), utilisateur final (hôpitaux et cliniques, entreprises pharmaceutiques et biotechnologiques, universités et recherche Instituts, laboratoires cliniques et autres), canal de distribution (commerce direct, vente au détail et autres)
|
|
Pays couverts
|
États-Unis, Canada et Mexique
|
|
Acteurs du marché couverts
|
Thermo Fisher Scientific Inc. (États-Unis), QIAGEN (Pays-Bas), Illumina, Inc. (États-Unis), Beckman Coulter, Inc. (États-Unis), Eurofins Scientific (Luxembourg), ExoDx (une partie de Bio-Techne) (États-Unis), FOUNDATION MEDICINE, INC. (filiale de F. Hoffmann-La Roche Ltd) (États-Unis), GeneFirst Limited (États-Unis), Meridian (États-Unis), Merck KGaA (Allemagne), SOPHiA GENETICS (États-Unis), Azenta US Inc. (États-Unis ), CD Genomics (États-Unis), Twist Bioscience (États-Unis), PerkinElmer Genomics (une filiale de PerkinElmer Inc.) (États-Unis), GeneDx, LLC (États-Unis), Psomagen (États-Unis) et Integrated DNA Technologies, Inc. (États-Unis) entre autres.
|
|
Points de données couverts dans le rapport
|
En plus des informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie d'experts, l'épidémiologie des patients, une analyse du pipeline, une analyse des prix, et le cadre réglementaire.
|
Analyse sectorielle :
Le marché nord-américain du séquençage de l’exome est segmenté en fonction des composants, des produits et services, des applications, des utilisateurs finaux et des canaux de distribution.
- Sur la base des composants, le marché nord-américain du séquençage de l’exome entier (WES) est segmenté en séquençage de deuxième génération et en séquençage de troisième génération.
En 2023, le segment du séquençage de deuxième génération du segment des composants devrait dominer le marché
En 2023, le segment du séquençage de deuxième génération devrait dominer le marché avec une part de marché de 80,64 % car il constitue le composant majeur des procédures de séquençage de l’exome entier.
- Sur la base des produits et des services, le marché nord-américain du séquençage complet de l’exome (WES) est segmenté en systèmes, kits et services.
En 2023, le segment des systèmes du segment des produits et services devrait dominer le marché.
En 2023, le segment des systèmes devrait dominer le marché avec une part de marché de 47,76 % en raison des avancées technologiques dans les systèmes de séquençage.
- Sur la base des applications, le marché nord-américain du séquençage de l’exome entier (WES) est segmenté en découverte et développement de médicaments, recherche agricole et animale, diagnostics, médecine personnalisée et autres. En 2023, le segment de la découverte et du développement de médicaments devrait dominer le marché avec une part de marché de 35,02 %.
- Sur la base de l’utilisateur final, le marché nord-américain du séquençage de l’exome entier (WES) est segmenté en sociétés pharmaceutiques et biotechnologiques, instituts universitaires et de recherche, hôpitaux, laboratoires cliniques et autres. En 2023, le segment des sociétés pharmaceutiques et biotechnologiques devrait dominer le marché avec 43,54 % de part de marché.
- Sur la base du canal de distribution, le marché nord-américain du séquençage de l’exome entier (WES) est segmenté en commerce direct, ventes au détail et autres. En 2023, le segment du commerce direct devrait dominer le marché avec une part de marché de 57,20 %.
Acteurs majeurs
Data Bridge Market Research reconnaît les sociétés suivantes comme les principaux acteurs du marché du séquençage de l’exome entier (WES) : Thermo Fisher Scientific Inc. (États-Unis), QIAGEN (Pays-Bas), Illumina, Inc. (États-Unis), Beckman Coulter, Inc. (États-Unis), Eurofins Scientific (Luxembourg), entre autres.
Développement du marché
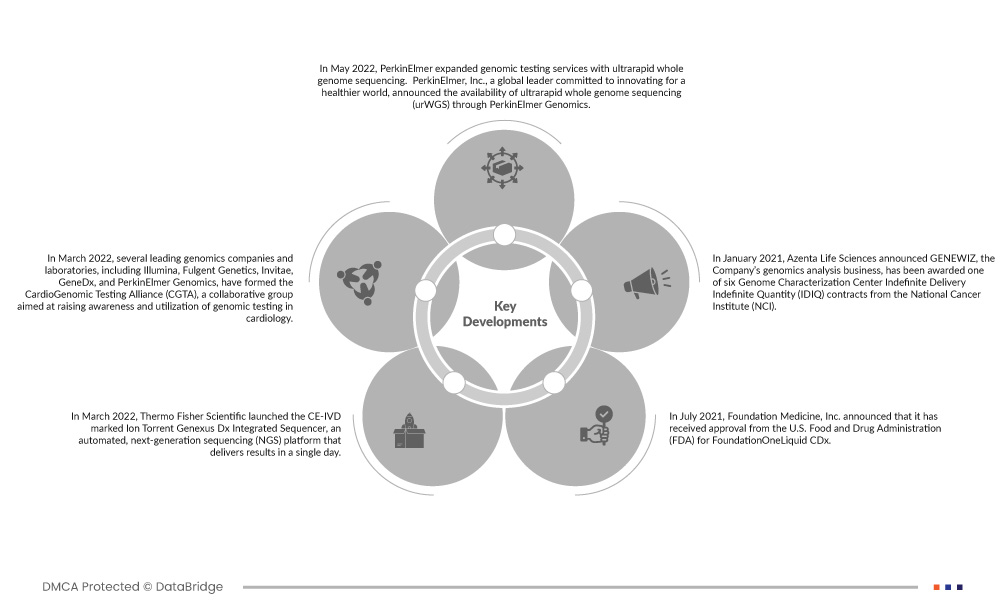
- En mai 2022, PerkinElmer a étendu ses services de tests génomiques avec le séquençage ultrarapide du génome entier. PerkinElmer, Inc., un leader mondial engagé à innover pour un monde plus sain, a annoncé la disponibilité du séquençage ultrarapide du génome entier (urWGS) via PerkinElmer Genomics. Cet ajout au portefeuille d'offres de séquençage du génome entier (WGS) de la société a fourni aux médecins des résultats complets et significatifs en cinq jours pour aider à éclairer la gestion clinique et à améliorer les résultats pour les patients gravement malades dans les unités de soins intensifs néonatals et pédiatriques. Cela a aidé l'entreprise à poursuivre ses recherches dans la division génomique.
- En mars 2022, plusieurs sociétés et laboratoires de génomique de premier plan, dont Illumina, Fulgent Genetics, Invitae, GeneDx et PerkinElmer Genomics, ont formé la CardioGenomic Testing Alliance (CGTA), un groupe collaboratif visant à sensibiliser et à utiliser les tests génomiques en cardiologie. La CGTA cherche à éduquer les prestataires de soins de santé et les autres parties prenantes sur la valeur de ces tests pour garantir le respect des directives existantes des sociétés médicales professionnelles, pour éclairer la gestion médicale et les tests en cascade, et pour améliorer les résultats cliniques. Cela a aidé l'entreprise à respecter les directives
- En mars 2022, Thermo Fisher Scientific a lancé le séquenceur intégré Ion Torrent Genexus Dx marqué CE-IVD, une plateforme automatisée de séquençage de nouvelle génération (NGS) qui fournit des résultats en une seule journée. Il est conçu pour être utilisé dans les laboratoires cliniques. Le système entièrement validé permet aux utilisateurs d'effectuer à la fois des tests de diagnostic et des recherches cliniques sur un seul instrument. Cela a aidé l'entreprise à faire progresser son portefeuille de produits.
- En juillet 2021, Foundation Medicine, Inc. a annoncé avoir reçu l'approbation de la Food and Drug Administration (FDA) des États-Unis pour l'utilisation de FoundationOneLiquid CDx comme diagnostic compagnon pour aider à identifier les patients présentant des sauts d'exon 14 MET (METex14) dans les cas métastatiques. cancer du poumon non à petites cellules (CPNPC) pour lequel un traitement par TABRECTA (capmatinib) peut être approprié. Cela a aidé l'entreprise à élargir son portefeuille
- En janvier 2021, Azenta Life Sciences a annoncé que GENEWIZ, l'activité d'analyse génomique de la société, avait remporté l'un des six contrats de centre de caractérisation du génome à livraison indéterminée (IDIQ) du National Cancer Institute (NCI). Le NCI, leader national de la recherche sur le cancer et membre des National Institutes of Health (NIH), a choisi GENEWIZ pour fournir le séquençage de l'exome entier à de nouveaux projets de recherche dans son Centre de génomique du cancer pendant trois ans. Cela a aidé l'entreprise à se concentrer sur le séquençage du génome
Analyse régionale
Géographiquement, les pays couverts dans l’ensemble du rapport sur le marché du séquençage de l’exome sont les États-Unis, le Canada et le Mexique.
Selon l’analyse de l’étude de marché Data Bridge :
Les États-Unis sont le pays dominant et à la croissance la plus rapide du monde. marché entier du séquençage de l’exome pendant le période de prévision 2023 - 2030
Les États-Unis devraient dominer l’ensemble du marché du séquençage de l’exome en raison de la demande accrue de thérapies ciblées au sein de la population et de diverses industries. Cela est également dû à la demande croissante de technologie WES dans les nouvelles applications.
Pour des informations plus détaillées sur le rapport sur le marché européen des dispositifs de chirurgie micro-invasive du glaucome (MIGS), cliquez ici –https://www.databridgemarketresearch.com/reports/north-america-whole-exome-sequencing-market