Asia-Pacific Scleroderma Therapeutics Market Segmentation, By Drug Class (Immunosuppressants, Endothelin Receptor Antagonists, Phosphodiesterase 5 Inhibitors, Calcium Channel Blockers, Prostacyclin Analogues, and Others), Disease Type (Localized and Systemic), Route of Administration (Oral, Injectable, and Topical), End User (Hospitals, Specialty Clinics, Homecare, and Others), and Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies)- Industry Trends and Forecast to 2032
Scleroderma Therapeutics Market Size
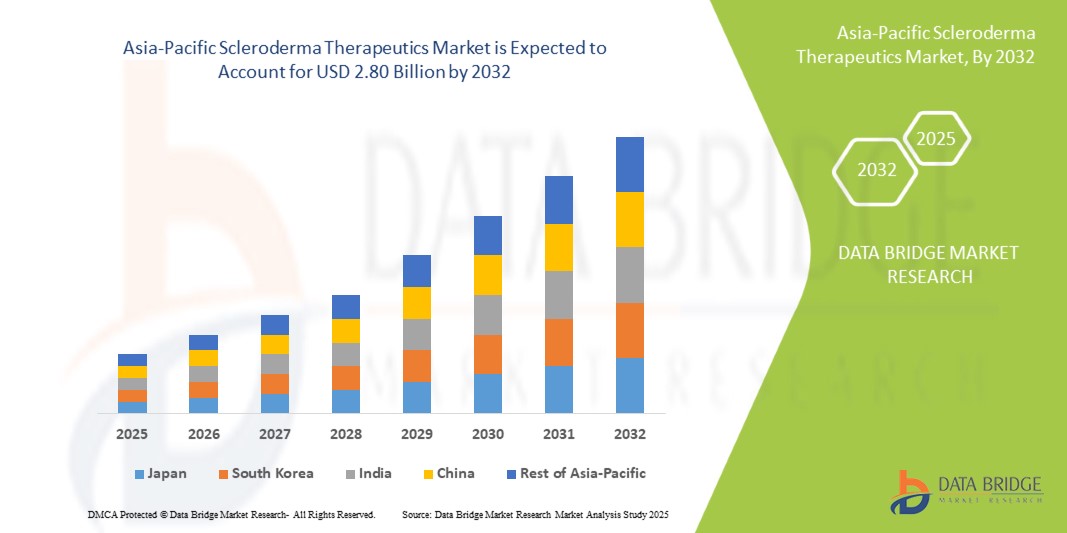
- The Asia-Pacific Scleroderma Therapeutics market size was valued atUSD 1.44 billion in 2024and is expected to reachUSD 2.80 billion by 2032, at aCAGR of 8.7%during the forecast period
- This growth is driven by increasing awareness about autoimmune disorders, rising incidence of systemic sclerosis, and improved access to targeted therapies across emerging Asian economies.
Scleroderma Therapeutics Market Analysis
- Scleroderma Therapeutics, also known as biotherapy or immunotherapy, uses living organisms or their components to treat diseases. These therapies have shown significant efficacy in managing cancer, autoimmune disorders, and genetic diseases due to their targeted action and reduced systemic side effects
- The demand for Scleroderma Therapeutics is significantly driven by rising incidences of cancer and autoimmune disorders, expanding approvals of biosimilars, and international investments in biotechnology innovation
- India is expected to dominate the Asia-Pacific Scleroderma Therapeutics market due to national rare disease programs, autoimmune disorder registries, and high treatment demand in systemic sclerosis.
- South Korea is expected to witness the highest CAGR in the Scleroderma Therapeutics market due to its growing biologics pipeline, clinical trial capacity, and government-backed healthcare innovation.
- Immunosuppressants segment is expected to hold the largest share of 42.5%, due to its widespread use in managing skin fibrosis and systemic complications. Expanding use of corticosteroids and methotrexate-based therapies is fueling demand in primary care settings.
Report Scope andScleroderma Therapeutics Market Segmentation
|
Attributes |
Scleroderma Therapeutics KeyMarket Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Scleroderma Therapeutics Market Trends
“Integration of Real-World Evidence in Drug Evaluation”
The use of real-world evidence (RWE) is increasingly influencing clinical and regulatory decisions in the Asia-Pacific Scleroderma Therapeutics market. Real-world data, derived from electronic health records, observational studies, and patient registries, provides critical insights into long-term treatment outcomes and safety profiles outside the controlled environment of clinical trials. Regulatory agencies in countries likeJapanandSouth Koreaare actively promoting the integration of patient-reported outcomes and RWE into therapeutic assessments to accelerate drug approval timelines, especially for rare and autoimmune conditions. These initiatives are enabling more accurate benefit-risk evaluations and paving the way for conditional approvals of novel biologics for scleroderma treatment.
Scleroderma Therapeutics Market Dynamics
Driver
“Growing Prevalence of Systemic Sclerosis and Early Diagnosis”
- The Asia-Pacific region is witnessing a growing burden of systemic sclerosis, driven by environmental triggers, genetic predispositions, and increased clinical awareness. Government bodies and rheumatology associations acrossIndia, China, and Australiaare initiating early screening and referral programs to combat diagnostic delays. The rise in trained rheumatologists, along with improved access to autoimmune testing in tier 2 and 3 cities, has led to higher detection rates. Early diagnosis is crucial to initiating treatment before irreversible organ damage occurs.
- For instance,
In 2024,India’s Ministry of Health and Family Welfarelaunched a national autoimmune disorder registry to track conditions like systemic sclerosis. This initiative has enhanced the identification of early-stage patients, supported regional treatment planning, and promoted personalized medicine approaches across public healthcare facilities.
Opportunity
“Emergence of Biologics for Scleroderma Lung Complications”
- Lung-related complications such as interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH) are among the leading causes of mortality in systemic sclerosis patients. The therapeutic landscape is rapidly evolving with the introduction of biologics that target fibrotic pathways, inflammatory cytokines, and vascular remodeling. Biologics likenintedanibandtocilizumabhave shown promise in slowing lung function decline and are being incorporated into treatment protocols across Asia-Pacific.
- For instance,
InNovember 2024,Boehringer Ingelheimannounced the expansion of its global ILD drug trial network to include clinical sites inIndia, South Korea, and Taiwan. This move aims to generate regional data to support regulatory approvals, improve representation of Asian patients in global trials, and enhance local treatment options.
Restraint/Challenge
“Limited Awareness and High Cost of Advanced Therapies”
- Despite advancements in therapeutics, awareness and accessibility remain major challenges, particularly in low- and middle-income countries across the Asia-Pacific region. Underdiagnosis of scleroderma in rural and semi-urban areas is common due to the lack of trained rheumatologists and limited availability of advanced diagnostic tools. Moreover, biologic therapies, while effective, are costly and often not covered under national insurance schemes, making them unaffordable for many patients.
- For instance,
In2023, a study published inThe Lancet Rheumatologyfocusing on scleroderma patients inIndiarevealed that the median time from symptom onset to diagnosis was18 months, primarily due to inadequate awareness and limited access to specialty care. Additionally, the high out-of-pocket expenses for biologics and immunomodulators have further hindered widespread adoption, necessitating policy reforms and the introduction of biosimilars to ensure affordability.
Scleroderma Therapeutics Market Scope
The market is segmented on the basis application, product type, technology, magnification type, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drug Class |
|
|
By Disease Type |
|
|
By Route of Administration |
|
|
By End User |
|
|
ByDistribution Channel
|
|
In 2025, the Immunosuppressants segment is projected to dominate the market with the largest share in drug class segment
In 2025, the Immunosuppressants segment is expected to hold the largest share of42.5%, due to its widespread use in managing skin fibrosis and systemic complications. Expanding use of corticosteroids and methotrexate-based therapies is fueling demand in primary care settings.
The Systemic Scleroderma segment is expected to account for the largest share during the forecast period in disease type segment
In 2025, the Systemic Scleroderma segment is anticipated to capture a63.4%share, driven by growing diagnosis of internal organ involvement and increased availability of systemic treatment options.
Scleroderma Therapeutics Market Regional Analysis
“India Holds the Largest Share in the Scleroderma Therapeutics Market”
- India is expected to dominate the Asia-Pacific Scleroderma Therapeutics market due to national rare disease programs, autoimmune disorder registries, and high treatment demand in systemic sclerosis.
- Additionally, India's large public health infrastructure and increasing affordability of generic immunosuppressants are boosting access to first-line therapies across tier 2 and tier 3 cities.
“South Korea is Projected to Register the HighestCAGR in the Scleroderma Therapeutics Market”
- South Korea is expected to witness the highest CAGR in the Scleroderma Therapeutics market due to its growing biologics pipeline, clinical trial capacity, and government-backed healthcare innovation.
- The country also benefits from strong academic-industry partnerships and digital health integration, which are accelerating early diagnosis and patient recruitment for rare disease research.
Scleroderma Therapeutics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Boehringer Ingelheim International GmbH (Germany)
- Pfizer Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Sanofi S.A. (France)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Bayer AG (Germany)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Gilead Sciences, Inc. (U.S.)
- Mitsubishi Tanabe Pharma Corporation (Japan)
- Actelion Pharmaceuticals Ltd (Switzerland)
Latest Developments in Asia-Pacific Scleroderma Therapeutics Market
- In February 2025, Mitsubishi Tanabe Pharma launched a new topical therapy for localized scleroderma in Japan, targeting pediatric patients.
- In January 2025, Roche expanded its Actemra (tocilizumab) scleroderma study to India and Indonesia as part of its APAC rare disease strategy.
- In October 2024, a multicenter trial evaluating sildenafil for digital ulcers in systemic sclerosis was launched across 5 Chinese hospitals.
- In March 2025, Pfizer Inc. partnered with the Japan Scleroderma Research Network (JSRN) to launch a post-marketing surveillance program evaluating long-term outcomes of immunosuppressants in systemic sclerosis patients, marking a strategic step towards real-world data collection and label expansion in the Japanese market.
- In April 2025, Roche initiated a multicenter Phase II clinical trial in Australia, Singapore, and South Korea for a novel antifibrotic monoclonal antibody targeting skin and lung fibrosis in systemic sclerosis, aiming to address unmet needs in severe scleroderma manifestations through precision biologics.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。