Europe Medical Device Testing Market
市场规模(十亿美元)
CAGR :
% 
 USD
1,226.46 Million
USD
2,499.53 Million
2021
2029
USD
1,226.46 Million
USD
2,499.53 Million
2021
2029
| 2022 –2029 | |
| USD 1,226.46 Million | |
| USD 2,499.53 Million | |
|
|
|
欧洲医疗器械测试市场,按服务类型(测试服务、检验服务和认证服务)、测试类型(物理测试、化学/生物测试、网络安全测试、微生物学和无菌测试等)、阶段(临床前和临床)、采购类型(内部和外包)、设备类别(I 类、II 类和 III 类)、产品(有源植入医疗器械、有源医疗器械、无源医疗器械、体外诊断医疗器械、眼科医疗器械、骨科和牙科医疗器械、血管医疗器械等)划分,行业趋势和预测至 2029 年
欧洲医疗器械测试市场分析与洞察
医疗器械测试是证明设备在使用中可靠且安全的过程。在新产品开发中,会应用广泛的设计验证测试。这包括性能测试、毒性、化学分析,有时还包括人为因素或临床测试。持续的质量保证测试通常更为有限。这通常包括尺寸检查、一些功能测试和包装验证。市场上有各种类型的医疗测试服务,例如检验服务、认证服务等

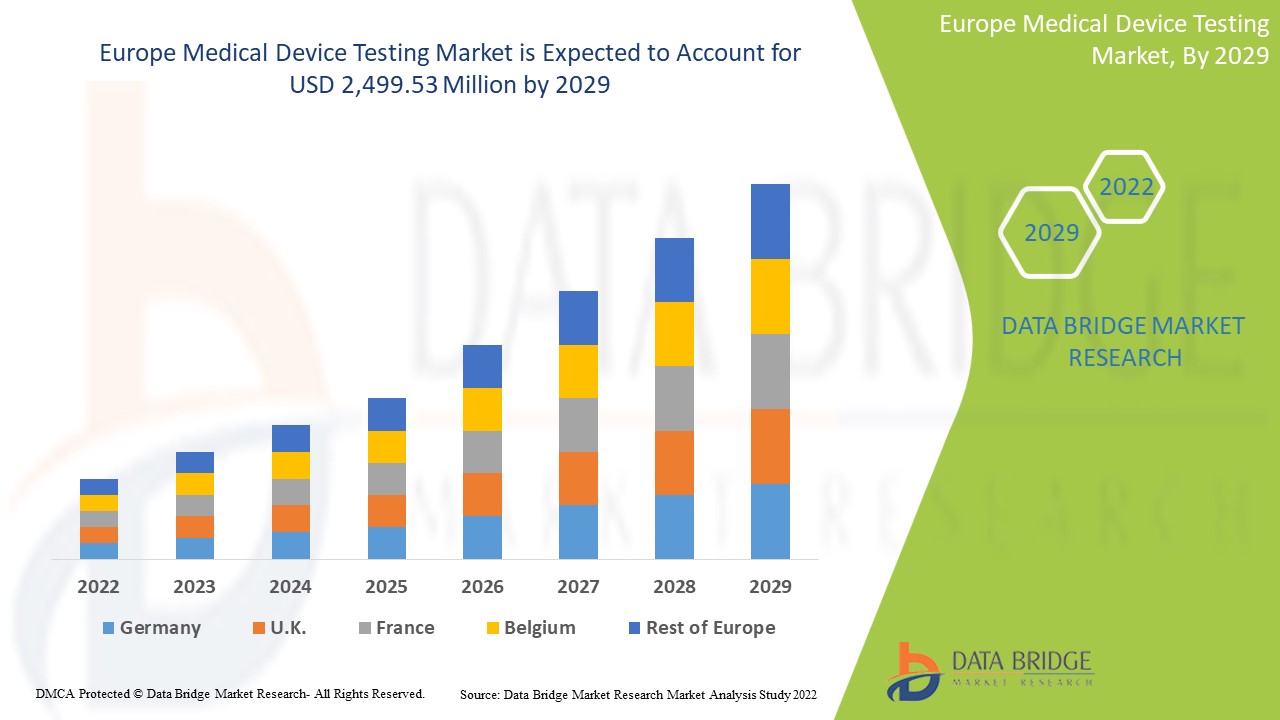
欧洲医疗器械测试市场预计将在 2022 年至 2029 年的预测期内增长。Data Bridge Market Research 分析称,在 2022 年至 2029 年的预测期内,该市场以 9.8% 的复合年增长率增长,预计将从 2021 年的 12.2646 亿美元达到 2029 年的 24.9953 亿美元。
|
报告指标 |
细节 |
|
预测期 |
2022 至 2029 年 |
|
基准年 |
2021 |
|
历史岁月 |
2020 (可定制为 2019-2014) |
|
定量单位 |
收入(百万美元),定价(美元) |
|
涵盖的领域 |
按服务类型(测试服务、检验服务和认证服务)、测试类型(物理测试、化学/生物测试、网络安全测试、微生物学和无菌测试等)、阶段(临床前和临床)、采购类型(内部和外包)、设备类别(I 类、II 类和 III 类)、产品(有源植入医疗器械、有源医疗器械、无源医疗器械、体外诊断医疗器械、眼科医疗器械、骨科和牙科医疗器械、血管医疗器械等) |
|
覆盖国家 |
德国、法国、英国、意大利、西班牙、荷兰、俄罗斯、瑞士、土耳其、比利时和欧洲其他地区 |
|
涵盖的市场参与者 |
Intertek Group plc、SGS SA、Bureau Veritas、TUV SUD、TUV Rheinland、UL LLC、North American Science Associates, LLC、Medistri SA、WuXi AppTec、NSF、Labcorp、Eurofins Scientific、Nelson Laboratories, LLC- A Sotera Health company、ITC ZLIN、Element Materials Technology、EndoLab Mechanical Engineering GmbH、Hohenstein、Medical Engineering Technologies Ltd.、Cigniti、IMR Test Labs 等 |
市场定义
医疗器械测试是证明设备在使用中可靠且安全的过程。在新产品开发中,会应用广泛的设计验证测试。这包括性能测试、毒性和化学分析,有时还包括人为因素甚至临床测试。持续的质量保证测试通常更为有限。这通常包括尺寸检查、一些功能测试和包装验证。市场上有各种类型的医疗测试服务,例如检验服务、认证服务等
医疗器械测试市场动态
驱动程序
- 创新和技术的升级
过去几年,医疗保健领域的技术发展速度大幅提升。医疗器械技术的进步有助于在疾病管理过程中实现无痛、简单的治疗。此外,医疗器械的创新和升级有助于准确、快速地诊断疾病。医疗器械的创新还为疾病治疗过程中基于技术的治疗工具提供了成本效益。此外,许多政府机构和医疗保健组织都在支持医学研究中心。这种支持的主要目的是加强欧洲的医疗保健创新
- 体外测试需求增加
体外诊断 (IVD) 是对从人体采集的血液或组织等样本进行的测试。体外诊断可以检测疾病或其他状况,并可用于监测一个人的整体健康状况,以帮助治愈、治疗或预防疾病。体外测试用于各种疾病检测,例如 HIV 感染、疟疾、肝炎等。此类疾病的流行率在欧洲国家迅速上升,导致对体外测试和各种医疗设备的需求不断增加。
机会
-
医疗支出增加
随着各国人民的可支配收入不断增加,全球医疗保健支出也随之增加。此外,为了满足人口需求,政府机构和医疗保健组织正在采取主动行动,加快医疗保健支出。医疗保健支出的增加同时帮助医疗保健机构近年来改善其医疗器械测试服务
此外,主要市场参与者采取的战略举措将为 2022-2029 年预测期内的医疗器械测试市场提供结构完整性和未来机遇。
克制/挑战
- 医疗技术行业竞争激烈
然而,医疗器械本地化发展的障碍和某些地区医疗器械的高成本可能会阻碍医疗器械的生产,从而阻碍市场的增长。此外,医疗技术行业的激烈竞争和海外认证的漫长准备时间可能是市场发展的挑战因素
本医疗器械测试市场报告详细介绍了最新发展、贸易法规、进出口分析、生产分析、价值链优化、市场份额、国内和本地市场参与者的影响,分析了新兴收入领域的机会、市场法规的变化、战略市场增长分析、市场规模、类别市场增长、应用领域和主导地位、产品批准、产品发布、地域扩展、市场技术创新。如需了解更多信息,请联系 Data Bridge Market Research 获取分析师简报,我们的团队将帮助您做出明智的市场决策,实现市场增长。
COVID-19 对医疗器械测试市场的影响
COVID-19 对市场产生了积极影响。这些年来,MRI 扫描仪、呼吸机等医疗设备的使用有所增加。因此,全球范围内各种设备的使用量大幅增加。因此,疫情对这个测试市场产生了积极影响。
近期发展
- 2021 年 4 月,TUV SUD 宣布已在 Medtec LIVE 上亮相,展示其作为医疗器械测试一站式服务商的能力。该公司的服务涵盖电气和功能安全、网络安全和软件、EMC 和生物相容性测试。TUV SUD 的专家在在线贸易展览和会议计划中进行了各种演讲、现场直播和电梯演讲。
欧洲医疗器械测试市场范围
欧洲医疗器械测试市场分为服务类型、测试类型、阶段、采购类型、设备类别和产品。这些细分市场之间的增长将帮助您分析行业中增长微弱的细分市场,并为用户提供有价值的市场概览和市场洞察,以便做出战略决策,确定核心市场应用。
服务类型
- 测试服务
- 检验服务
- 认证服务
根据服务类型,欧洲医疗器械测试市场分为测试服务、检验服务和认证服务。
测试类型
- 物理测试
- 化学/生物测试
- 网络安全测试
- 微生物学和无菌测试
- 其他的
根据测试类型,欧洲医疗器械测试市场分为物理测试、化学/生物测试、网络安全测试、微生物学、无菌测试等。
阶段
- 临床前
- 临床
根据阶段,欧洲医疗器械测试市场分为临床前和临床。
.采购类型
- 外包
- 内部
根据采购类型,欧洲医疗器械测试市场分为内部采购和外包采购。
设备类别
- 一级
- II 类
- III 级
根据设备类别,欧洲医疗器械测试市场分为 I 类、II 类和 III 类。
产品
- 有源植入医疗器械
- 有源医疗器械
- 无源医疗器械
- 体外诊断医疗器械
- 眼科医疗器械
- 骨科及牙科医疗设备
- 血管医疗器械
- 其他的
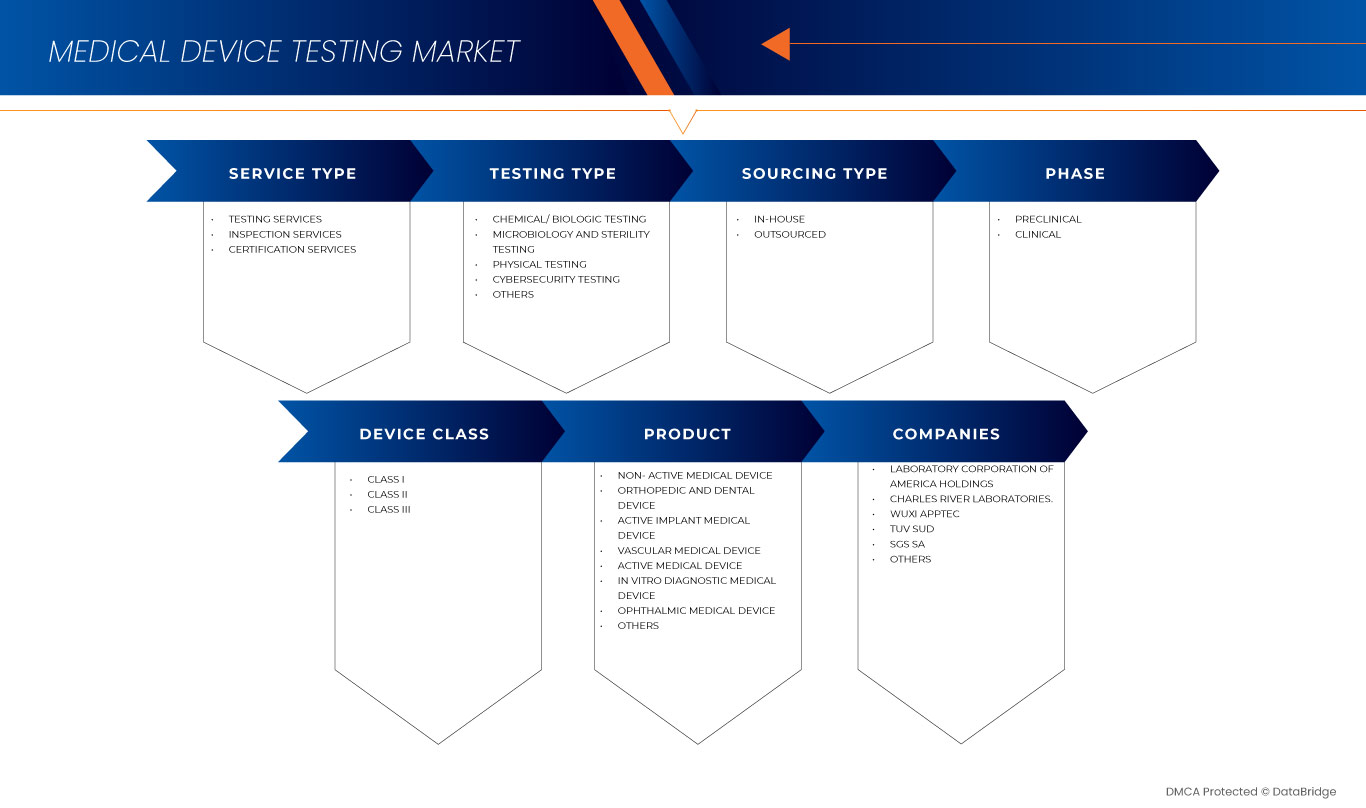
根据产品,欧洲医疗器械测试市场细分为有源植入医疗器械、有源医疗器械、无源医疗器械、体外诊断医疗器械、眼科医疗器械、骨科和牙科医疗器械、血管医疗器械等。
医疗器械测试市场区域分析/见解
对医疗器械测试市场进行了分析,并按国家、服务类型、测试类型、阶段、采购类型、设备类别和产品提供了市场规模见解和趋势。
欧洲地区涵盖的国家包括英国、德国、意大利、法国、西班牙、俄罗斯、荷兰、瑞士、土耳其、比利时和欧洲其他地区
德国在市场份额和市场收入方面占据医疗器械检测市场的主导地位,并将在预测期内继续保持主导地位。这是由于欧洲对体外测试的需求不断增加。
报告的国家部分还提供了影响单个市场因素和市场法规变化的信息,这些因素和变化会影响市场的当前和未来趋势。新旧销售、国家人口统计、疾病流行病学和进出口关税等数据点是预测单个国家市场情况的一些主要指标。此外,在对国家数据进行预测分析时,还考虑了全球品牌的存在和可用性以及它们因本土和国内品牌的激烈竞争而面临的挑战以及销售渠道的影响。
竞争格局和医疗器械测试市场份额分析
医疗器械测试市场竞争格局提供了竞争对手的详细信息。详细信息包括公司概况、公司财务状况、产生的收入、市场潜力、研发投资、新市场计划、全球影响力、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度以及应用主导地位。以上提供的数据点仅与公司对医疗器械测试市场的关注有关。
医疗器械测试市场的一些主要参与者包括 Intertek Group plc、SGS SA、Bureau Veritas、TUV SUD、TUV Rheinland、UL LLC、North American Science Associates, LLC、Medistri SA、WuXi AppTec、NSF、Labcorp、Eurofins Scientific、Nelson Laboratories, LLC- A Sotera Health company、ITC ZLIN、Element Materials Technology、EndoLab Mechanical Engineering GmbH、Hohenstein、Medical Engineering Technologies Ltd.、Cigniti、IMR Test Labs 等。
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析以及主要(行业专家)验证。除此之外,数据模型还包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、公司市场份额分析、测量标准、欧洲与地区和供应商份额分析。如有进一步询问,请要求分析师致电。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE MEDICAL DEVICE TESTING MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICE TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
5 EUROPE MEDICAL DEVICE TESTING MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING NEED FOR VERIFICATION VALIDATION OF MEDICAL DEVICES
6.1.2 INCREASING DEMAND FOR IN-VITRO TESTS
6.1.3 ESCALATION IN INNOVATION AND TECHNOLOGIES
6.2 RESTRAINTS
6.2.1 BARRIERS TO THE LOCAL DEVELOPMENT OF MEDICAL DEVICES
6.2.2 HIGH COST OF MEDICAL DEVICES
6.3 OPPORTUNITIES
6.3.1 RISE IN HEALTHCARE EXPENDITURE
6.3.2 DEVELOPMENT IN AI AND IOT IN VARIOUS MEDICAL DEVICES
6.3.3 STRATEGIC INITIATIVES OF KEY PLAYERS
6.4 CHALLENGES
6.4.1 HIGH COMPETITION IN MEDICAL TECHNOLOGY INDUSTRY
6.4.2 LONG LEAD TIME FOR OVERSEAS QUALIFICATION
7 EUROPE MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE
7.1 OVERVIEW
7.2 TESTING SERVICES
7.3 INSPECTION SERVICES
7.4 CERTIFICATION SERVICES
8 EUROPE MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE
8.1 OVERVIEW
8.2 CHEMICAL/BIOLOGICAL TESTING
8.3 MICROBIOLOGY AND STERILITY TESTING
8.3.1 STERILITY TEST & VALIDATION
8.3.2 BIO BURDEN DETERMINATION
8.3.3 ANTIMICROBIAL ACTIVITY TESTING
8.3.4 PYROGEN & ENDOTOXIN TESTING
8.3.5 OTHERS
8.4 PHYSICAL TESTING
8.4.1 ELECTRICAL SAFETY TESTING
8.4.2 FUNCTIONAL SAFETY TESTING
8.4.3 EMC TESTING
8.4.4 ENVIRONMENTAL TESTING
8.4.5 OTHERS
8.5 CYBERSECURITY TESTING
8.6 OTHERS
9 EUROPE MEDICAL DEVICE TESTING MARKET, BY PHASE
9.1 OVERVIEW
9.2 PRECLINICAL
9.3 CLINICAL
10 EUROPE MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE
10.1 OVERVIEW
10.2 OUTSOURCED
10.3 IN-HOUSE
11 EUROPE MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS
11.1 OVERVIEW
11.2 CLASS I
11.3 CLASS III
11.4 CLASS II
12 EUROPE MEDICAL DEVICE TESTING MARKET, BY PRODUCT
12.1 OVERVIEW
12.2 NON-ACTIVE MEDICAL DEVICE
12.3 ORTHOPEDIC AND DENTAL MEDICAL DEVICE
12.4 ACTIVE IMPLANT MEDICAL DEVICE
12.5 VASCULAR MEDICAL DEVICE
12.6 ACTIVE MEDICAL DEVICE
12.7 IN-VITRO DIAGNOSTICS MEDICAL DEVICE
12.8 OPTHALMIC MEDICAL DEVICE
12.9 OTHERS
13 EUROPE MEDICAL DEVICE TESTING MARKET, BY GEOGRAPHY
13.1 EUROPE
13.1.1 GERMANY
13.1.2 U.K.
13.1.3 FRANCE
13.1.4 ITALY
13.1.5 SPAIN
13.1.6 RUSSIA
13.1.7 NETHERLANDS
13.1.8 SWITZERLAND
13.1.9 TURKEY
13.1.10 BELGIUM
13.1.11 REST OF EUROPE
14 EUROPE MEDICAL DEVICE TESTING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 LABCORP
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 CHARLES RIVER LABORATORIES.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 TUV SUD
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 WUXI APPTEC
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 SGS SA
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 NORTH AMERICAN SCIENCE ASSOCIATES, LLC
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENTS
16.7 HOHENSTEIN
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 ARBRO PHARMACEUTICALS PRIVATE LIMITED & AURIGA RESEARCH PRIVATE LIMITED
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 BIOMEDICAL DEVICE LABS
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENTS
16.1 BIONEEDS
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 BUREAU VERITAS
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENTS
16.12 CIGNITI
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENTS
16.13 ELEMENT MATERIALS TECHNOLOGY
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 ENDOLAB MECHANICAL ENGINEERING GMBH
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 EUROFINS SCIENTIFIC
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GATEWAY ANALYTICAL.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 IMR TEST LABS
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 INTERTEK GROUP PLC
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 PRODUCT PORTFOLIO
16.18.4 RECENT DEVELOPMENTS
16.19 ITC ZLIN
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENTS
16.2 MEDICAL ENGINEERING TECHNOLOGIES LTD.
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 MEDISTRI SA
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENTS
16.22 NELSON LABORATORIES, LLC- A SOTERA HEALTH COMPANY
16.22.1 COMPANY SNAPSHOT
16.22.2 REVENUE ANALYSIS
16.22.3 PRODUCT PORTFOLIO
16.22.4 RECENT DEVELOPMENTS
16.23 NSF.
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENTS
16.24 PACE
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENTS
16.25 Q LABORATORIES
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENTS
16.26 TUV RHEINLAND
16.26.1 COMPANY SNAPSHOT
16.26.2 REVENUE ANALYSIS
16.26.3 PRODUCT PORTFOLIO
16.26.4 RECENT DEVELOPMENTS
16.27 UL LLC
16.27.1 COMPANY SNAPSHOT
16.27.2 PRODUCT PORTFOLIO
16.27.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
表格列表
TABLE 1 FDA REGULATIONS BASED ON DEVICES TYPE
TABLE 2 PRICES OF ESSENTIAL MEDICAL DEVICES
TABLE 3 EUROPE MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 4 EUROPE TESTING SERVICES IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE INSPECTION SERVICES IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE CERTIFICATION SERVICES IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 EUROPE MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 8 EUROPE CHEMICAL/BIOLOGICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 11 EUROPE PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 13 EUROPE CYBERSECURITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE OTHERS IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 16 EUROPE PRECLINICAL IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE CLINICAL IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 19 EUROPE OUTSOURCED IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE IN-HOUSE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 22 EUROPE CLASS I IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE CLASS III IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE CLASS II IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 26 EUROPE NON-ACTIVE MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE ORTHOPEDIC AND DENTAL MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE ACTIVE IMPLANT MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE VASCULAR MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE ACTIVE MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE IN-VITRO MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 EUROPE OPTHALMIC MEDICAL DEVICE IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE OTHERS IN MEDICAL DEVICE TESTING MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE MEDICAL DEVICE TESTING MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 35 EUROPE MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 36 EUROPE MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 37 EUROPE MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 38 EUROPE PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 39 EUROPE MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 40 EUROPE MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 41 EUROPE MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 42 EUROPE MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 43 GERMANY MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 44 GERMANY MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 45 GERMANY MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 46 GERMANY PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 47 GERMANY MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 48 GERMANY MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 49 GERMANY MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 50 GERMANY MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 51 U.K. MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 52 U.K. MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 53 U.K. MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 54 U.K. PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 55 U.K. MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 56 U.K. MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 57 U.K. MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 58 U.K. MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 59 FRANCE MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 60 FRANCE MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 61 FRANCE MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 62 FRANCE PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 63 FRANCE MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 64 FRANCE MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 65 FRANCE MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 66 FRANCE MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 67 ITALY MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 68 ITALY MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 69 ITALY MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 70 ITALY PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 71 ITALY MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 72 ITALY MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 73 ITALY MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 74 ITALY MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 75 SPAIN MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 76 SPAIN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 77 SPAIN MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 78 SPAIN PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 79 SPAIN MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 80 SPAIN MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 81 SPAIN MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 82 SPAIN MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 83 RUSSIA MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 84 RUSSIA MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 85 RUSSIA MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 86 RUSSIA PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 87 RUSSIA MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 88 RUSSIA MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 89 RUSSIA MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 90 RUSSIA MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 91 NETHERLANDS MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 92 NETHERLANDS MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 93 NETHERLANDS MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 94 NETHERLANDS PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 95 NETHERLANDS MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 96 NETHERLANDS MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 97 NETHERLANDS MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 98 NETHERLANDS MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 99 SWITZERLAND MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 100 SWITZERLAND MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 101 SWITZERLAND MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 102 SWITZERLAND PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 103 SWITZERLAND MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 104 SWITZERLAND MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 105 SWITZERLAND MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 106 SWITZERLAND MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 107 TURKEY MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 108 TURKEY MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 109 TURKEY MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 110 TURKEY PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 111 TURKEY MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 112 TURKEY MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 113 TURKEY MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 114 TURKEY MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 115 BELGIUM MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
TABLE 116 BELGIUM MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 117 BELGIUM MICROBIOLOGY AND STERILITY TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 118 BELGIUM PHYSICAL TESTING IN MEDICAL DEVICE TESTING MARKET, BY TESTING TYPE, 2020-2029 (USD MILLION)
TABLE 119 BELGIUM MEDICAL DEVICE TESTING MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 120 BELGIUM MEDICAL DEVICE TESTING MARKET, BY SOURCING TYPE, 2020-2029 (USD MILLION)
TABLE 121 BELGIUM MEDICAL DEVICE TESTING MARKET, BY DEVICE CLASS, 2020-2029 (USD MILLION)
TABLE 122 BELGIUM MEDICAL DEVICE TESTING MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 123 REST OF EUROPE MEDICAL DEVICE TESTING MARKET, BY SERVICE TYPE, 2020-2029 (USD MILLION)
图片列表
FIGURE 1 EUROPE MEDICAL DEVICE TESTING MARKET: SEGMENTATION
FIGURE 2 EUROPE MEDICAL DEVICE TESTING MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE MEDICAL DEVICE TESTING MARKET: DROC ANALYSIS
FIGURE 4 EUROPE MEDICAL DEVICE TESTING MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE MEDICAL DEVICE TESTING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE MEDICAL DEVICE TESTING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE MEDICAL DEVICE TESTING MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE MEDICAL DEVICE TESTING MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 EUROPE MEDICAL DEVICE TESTING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE MEDICAL DEVICE TESTING MARKET: SEGMENTATION
FIGURE 11 INCREASING DEMAND FOR IN-VITRO TESTS AND DEVELOPMENT IN AI AND IOT IN VARIOUS MEDICAL DEVICES ARE EXPECTED TO DRIVE THE EUROPE MEDICAL DEVICE TESTING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 TESTING SERVICES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE MEDICAL DEVICE TESTING MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE MEDICAL DEVICE TESTINGMARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE MEDICAL DEVICE TESTING MARKET
FIGURE 15 EUROPE MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, 2021
FIGURE 16 EUROPE MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, 2022-2029 (USD MILLION)
FIGURE 17 EUROPE MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, CAGR (2022-2029)
FIGURE 18 EUROPE MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE, LIFELINE CURVE
FIGURE 19 EUROPE MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, 2021
FIGURE 20 EUROPE MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, 2022-2029 (USD MILLION)
FIGURE 21 EUROPE MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, CAGR (2022-2029)
FIGURE 22 EUROPE MEDICAL DEVICE TESTING MARKET: BY TESTING TYPE, LIFELINE CURVE
FIGURE 23 EUROPE MEDICAL DEVICE TESTING MARKET: BY PHASE, 2021
FIGURE 24 EUROPE MEDICAL DEVICE TESTING MARKET: BY PHASE, 2022-2029 (USD MILLION)
FIGURE 25 EUROPE MEDICAL DEVICE TESTING MARKET: BY PHASE, CAGR (2022-2029)
FIGURE 26 EUROPE MEDICAL DEVICE TESTING MARKET: BY PHASE, LIFELINE CURVE
FIGURE 27 EUROPE MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, 2021
FIGURE 28 EUROPE MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, 2022-2029 (USD MILLION)
FIGURE 29 EUROPE MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, CAGR (2022-2029)
FIGURE 30 EUROPE MEDICAL DEVICE TESTING MARKET: BY SOURCING TYPE, LIFELINE CURVE
FIGURE 31 EUROPE MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, 2021
FIGURE 32 EUROPE MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, 2022-2029 (USD MILLION)
FIGURE 33 EUROPE MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, CAGR (2022-2029)
FIGURE 34 EUROPE MEDICAL DEVICE TESTING MARKET: BY DEVICE CLASS, LIFELINE CURVE
FIGURE 35 EUROPE MEDICAL DEVICE TESTING MARKET: BY PRODUCT, 2021
FIGURE 36 EUROPE MEDICAL DEVICE TESTING MARKET: BY PRODUCT, 2022-2029 (USD MILLION)
FIGURE 37 EUROPE MEDICAL DEVICE TESTING MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 38 EUROPE MEDICAL DEVICE TESTING MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 39 EUROPE MEDICAL DEVICE TESTING MARKET: SNAPSHOT (2021)
FIGURE 40 EUROPE MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2021)
FIGURE 41 EUROPE MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 42 EUROPE MEDICAL DEVICE TESTING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 43 EUROPE MEDICAL DEVICE TESTING MARKET: BY SERVICE TYPE (2022-2029)
FIGURE 44 EUROPE MEDICAL DEVICE TESTING MARKET: COMPANY SHARE 2021 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。













