美国骨坏死市场,按疾病类型(创伤性、非创伤性和特发性)、类型(诊断和治疗)、分期(第 1 期、第 2 期、第 3 期和第 4 期)、部位(髋关节/股骨头坏死、膝关节坏死、肩关节坏死、距骨坏死、月骨坏死、舟骨坏死等)、药物类型(品牌药和仿制药)、处方(非处方药和处方药)、性别(男性和女性)、年龄(儿童、成人和老年人)、最终用户(医院、专科诊所、骨科诊所、门诊中心等)、分销渠道(直接招标和零售销售)划分 - 行业趋势和预测到 2030 年。
美国骨坏死市场分析与洞察
美国人口中创伤性损伤的增加预计将推动美国骨坏死市场的增长。政府不断增加的药物研发和相关研究计划的计划和资金也促进了市场的增长。在这一关键时期,主要市场参与者专注于各种服务的推出和批准。此外,用于诊断骨坏死的成像技术的进步也在预测期内推动市场的增长。
由于市场参与者数量的增加以及先进技术诊断和手术设备的可用性,预计美国骨坏死市场将在预测年内增长。先进技术领域的不断发展有望进一步促进市场增长。然而,生产和商业化治疗药物以及用于诊断和手术的医疗器械的严格规定等困难预计将在预测期内抑制市场增长。
各公司都在采取举措,逐步推动市场增长:
例如,
- 2021 年 1 月,史赛克宣布收购 OrthoSensor,后者是一家将数字技术和大数据应用于全关节置换的市场参与者。此次收购将帮助该公司购买 OrthoSensor 的数字技术,用于骨坏死的诊断和治疗技术。
- 2020 年 3 月,Sciegen Pharmaceuticals 获得了萘普生片 USP 250mg、375mg 和 500mg 的 ANDA 批准。该批准将进一步帮助公司扩大产品组合。
Data Bridge Market Research分析,美国骨坏死市场预计到2030年将达到843,039.67万美元的价值,预测期内的复合年增长率为5.9%。
|
报告指标 |
细节 |
|
预测期 |
2023 至 2030 年 |
|
基准年 |
2022 |
|
历史岁月 |
2021 (可定制为 2015-2020) |
|
定量单位 |
收入(千)、销量(台)、定价(美元) |
|
涵盖的领域 |
疾病类型(创伤性、非创伤性和特发性)、类型(诊断和治疗)、阶段(第 1 阶段、第 2 阶段、第 3 阶段和第 4 阶段)、位置(髋骨坏死/股骨头坏死、膝骨坏死、肩骨坏死、距骨坏死、月骨坏死、舟骨坏死等)、药物类型(品牌和仿制药)、处方(非处方药和处方药)、性别(男性和女性)、年龄(儿童、成人和老年人)、最终用户(医院、专科诊所、骨科诊所、门诊中心等)、分销渠道(直接招标和零售销售) |
|
覆盖国家 |
我们 |
|
涵盖的市场参与者 |
Sciegen Pharmaceuticals、Almatica Pharma、Dr. Reddy's Laboratories Ltd.、Bayer AG、Pfizer Inc.、Haleon Group of Companies、Zimmer Biomet、Stryker、Teva Pharmaceuticals USA, Inc.(Teva Pharmaceutical Industries Ltd. 的子公司)和 Arthrex, Inc. 等。 |
美国骨坏死市场定义
骨坏死,也称为缺血性坏死或无菌性坏死,是一种由于血液供应不足而导致骨组织死亡或恶化的疾病。它可以发生在身体的各个骨骼中,但最常见的是髋关节。血流受阻使骨细胞缺乏必需的氧气和营养,导致其死亡,随后对受影响的骨骼造成损害。结果,骨骼结构变弱,无法承受正常压力,最终可能塌陷或变形。骨坏死可引起疼痛、活动范围受限和功能障碍。骨坏死的常见原因包括创伤、使用皮质类固醇、过量饮酒、某些医疗条件以及影响血液循环的潜在因素。早期诊断和适当的治疗对于防止进一步的骨损伤和保持关节功能至关重要。骨坏死的治疗方案可能包括保守措施来控制症状和减缓疾病进展,以及手术干预来缓解疼痛和恢复关节功能。
美国骨坏死市场动态
本节旨在了解市场驱动因素、优势、机遇、限制和挑战。下面将详细讨论所有这些内容:
司机
- 创伤性损伤不断增加
美国创伤性损伤数量的增加是美国骨坏死市场的重要驱动力。近年来,由事故、运动相关事件、跌倒和工作场所受伤等各种因素造成的创伤性损伤变得越来越普遍。这些损伤会严重影响骨骼健康,常常导致骨折、脱臼和关节不稳定。当受伤期间受影响骨骼的血液供应受损时,就为骨坏死的潜在发展奠定了基础。受损的血管会破坏骨组织所需的氧气和营养供应,导致骨细胞死亡。创伤后骨坏死是严重创伤性损伤的一个众所周知的后果。不及时诊断和治疗会导致关节塌陷、慢性疼痛和关节功能下降等并发症。
创伤性损伤会破坏向骨组织输送氧气和营养的血管。这种破坏会导致血流减少或阻塞,从而导致细胞死亡和骨坏死。此外,创伤还会增加骨骼或关节的压力,影响血流并导致缺血。由此导致的供血不足和细胞损伤会引发炎症反应,进一步加剧病情。道路和运动相关事故的数量不断增加,导致骨坏死的发病率上升。
克制
- 骨坏死诊断和治疗费用高昂
诊断技术和治疗方案的进步改善了患者的治疗效果。然而,治疗骨坏死的经济负担仍然是患者、医疗服务提供者和医疗保健系统面临的重大挑战。决定骨坏死诊断和治疗费用的关键因素包括确定的医疗机构或诊所、治疗区域、复杂程度以及所选专家等。
准确诊断骨坏死通常需要专门的成像技术,例如磁共振成像 (MRI)、计算机断层扫描 (CT) 或骨扫描。这些诊断程序费用昂贵,需要专门的设备、训练有素的人员和熟练的放射科医生的解释。这些成像程序的高成本限制了及时准确的诊断,导致治疗延误和潜在的并发症。治疗费用会相应增加,因此高昂的治疗费用可能会阻碍市场需求。
机会
-
骨坏死医疗技术的进展
医疗技术的进步为美国骨坏死市场带来了重大机遇。这些创新提高了诊断的准确性,提供了再生治疗选择,实现了微创手术,促进了个性化护理,并改善了患者的治疗效果。持续投资研发、行业与医疗保健专业人员之间的合作以及对创新技术的监管支持对于充分利用医疗进步的潜力并推动骨坏死的进展至关重要。因此,预计将成为市场的一个机会。
挑战
- 治疗选择有限
美国骨坏死市场缺乏治疗替代方案,这妨碍了患者获得最佳护理和治疗效果。骨坏死,通常称为缺血性坏死,是一种因血流不足而导致骨组织坏死的疾病。虽然有多种治疗方案,但选择通常受到骨坏死阶段、位置和潜在病因的限制。
最新动态
- 2023 年 1 月,Zimmer Biomet 宣布已达成最终协议,以 15.5 万美元的价格收购专注于软组织愈合的私营医疗器械公司 Embody, Inc.。此次收购包括 Embody 完整的基于胶原蛋白的生物整合解决方案组合,以支持最具挑战性的骨科软组织损伤的愈合——包括用于肌腱愈合的 TAPESTRY 生物整合植入物和用于肩袖修复的首批关节镜植入系统之一 TAPESTRY RC。此次收购将有助于该公司的产品组合和业务扩展。
- 2022 年 7 月,Haleon 集团公司宣布完成消费者保健业务从葛兰素史克集团的拆分,成立 Haleon 集团。此次拆分有助于该公司控制整个消费者保健业务并扩大业务。
美国骨坏死市场范围
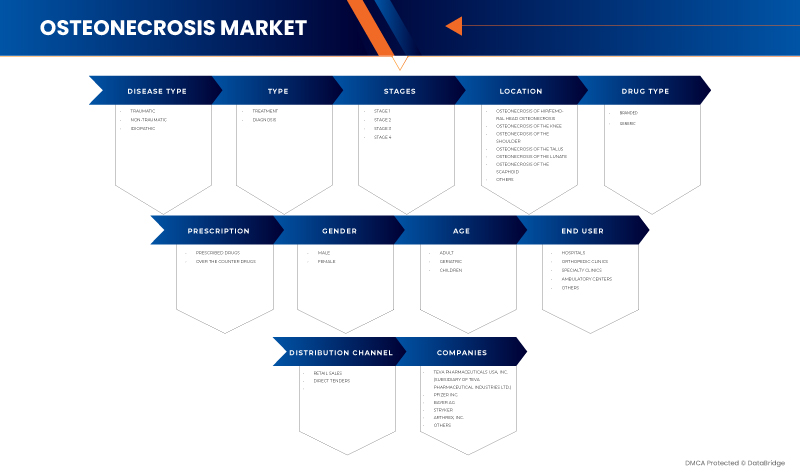
美国骨坏死市场根据疾病类型、类型、阶段、位置、药物类型、处方、性别、年龄、最终用户和分销渠道分为十个值得注意的细分市场。细分市场之间的增长有助于您分析利基增长领域和进入市场的策略,并确定您的核心应用领域和目标市场的差异。
疾病类型
- 创伤性
- 非创伤性
- 特发性
根据疾病类型,市场分为创伤性、非创伤性和特发性。
类型
- 诊断
- 治疗
根据类型,市场分为诊断和治疗。
阶段
- 第一阶段
- 第 2 阶段
- 第 3 阶段
- 第 4 阶段
根据阶段,市场分为第 1 阶段、第 2 阶段、第 3 阶段和第 4 阶段。
地点
- 髋骨坏死/股骨头坏死
- 膝关节骨坏死
- 肩关节骨坏死
- 距骨坏死
- 月骨坏死
- 舟骨坏死
- 其他的
根据地理位置,市场分为髋关节/股骨头坏死、膝关节坏死、肩关节坏死、距骨坏死、月骨坏死、舟骨坏死等。
药物类型
- 品牌
- 通用的
根据药品类型,市场分为品牌药品和仿制药。
处方
- 非处方药
- 处方药
根据处方,市场分为非处方药和处方药。
性别
- 男性
- 女性
根据性别,市场分为男性和女性。
年龄
- 孩子们
- 成人
- 老年
根据年龄,市场分为儿童、成人和老年人。
最终用户
- 医院
- 专科诊所
- 骨科诊所
- 流动中心
- 其他的
根据最终用户,市场分为医院、专科诊所、骨科诊所、门诊中心和其他。
分销渠道
- 直接招标
- 零售销售
根据分销渠道,市场分为直接招标和零售。
美国骨坏死市场分析/见解
根据疾病类型、类型、阶段、位置、药物类型、处方、性别、年龄、最终用户和分销渠道,美国骨坏死市场分为十个显著部分。
本市场报告涵盖的国家/地区 美国
报告的国家部分还提供了影响单个市场因素和国内市场监管变化,这些因素和变化会影响市场的当前和未来趋势。新销售、替代销售、国家人口统计、监管法案和进出口关税等数据点是用于预测单个国家市场情景的一些主要指标。此外,在对国家数据进行预测分析时,还考虑了美国品牌的存在和可用性以及它们因来自本地和国内品牌的激烈或稀少竞争而面临的挑战,以及销售渠道的影响。
竞争格局和美国骨坏死市场份额分析
美国骨坏死市场竞争格局按竞争对手提供详细信息。详细信息包括公司概况、公司财务状况、产生的收入、市场潜力、研发投资、新市场计划、生产基地和设施、公司优势和劣势、产品发布、产品批准、产品宽度和广度、应用主导地位以及产品类型生命线曲线。以上提供的数据点仅与公司对美国骨坏死市场的关注有关。
美国骨坏死市场的一些主要市场参与者包括 Sciegen Pharmaceuticals、Almatica Pharma、Dr. Reddy's Laboratories Ltd.、Bayer AG、Pfizer Inc.、Haleon Group of Companies、Zimmer Biomet、Stryker、Teva Pharmaceuticals USA, Inc.(Teva Pharmaceutical Industries Ltd. 的子公司)和 Arthrex, Inc. 等。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE U.S. OSTEONECROSIS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 DISEASE TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 INDUSTRY INSIGHTS
4.3.1 DEMOGRAPHIC TRENDS: IMAPCTS ON ALL INCIDENCE RATES
4.3.2 PATIENT FLOW DIAGRAM
4.3.3 KEY PRICING STRATEGIES
4.4 HEALTHCARE ECONOMY
4.4.1 HEALTHCARE EXPENDITURE
4.4.2 CAPITAL EXPENDITURE
4.4.3 CAPEX TRENDS
4.4.4 CAPEX ALLOCATION
4.4.5 FUNDING SOURCES
4.4.6 INDUSTRY BENCHMARKS
4.4.7 GDP RATIO IN OVERALL GDP
4.4.8 HEALTHCARE SYSTEM STRUCTURE
4.4.9 GOVERNMENT POLICIES
4.4.10 ECONOMIC DEVELOPMENT
4.5 PIPELINE ANALYSIS
4.6 REIMBURSEMENT FRAMEWORK
4.7 TECHNOLOGY ROADMAP
4.8 VALUE CHAIN ANALYSIS
5 EPIDEMIOLOGY
6 REGULATIONS
6.1 REGULATORY APPROVAL PROCESS AND PATHWAYS:
6.2 LICENSING AND REGISTRATION:
6.3 POST-MARKETING SURVEILLANCE:
6.4 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES:
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 INCREASING NUMBER OF TRAUMATIC INJURIES
7.1.2 INCREASING CONSUMPTION OF STEROIDS AND RELATED DRUGS
7.1.3 INCREASING INCIDENCE OF OSTEONECROSIS
7.2 RESTRAINTS
7.2.1 HIGH COST ASSOCIATED WITH THE DIAGNOSIS AND TREATMENT OF OSTEONECROSIS
7.2.2 PROGNOSTIC AND DIAGNOSTIC CHALLENGES
7.3 OPPORTUNITIES
7.3.1 ADVANCEMENTS IN MEDICAL TECHNOLOGY FOR OSTEONECROSIS
7.3.2 INCREASING REIMBURSEMENTS SCENARIOS
7.4 CHALLENGES
7.4.1 AVAILABILITY OF LIMITED TREATMENT OPTIONS
7.4.2 CHALLENGING LONG-TERM MANAGEMENT AND FOLLOW UP
8 U.S. OSTEONECROSIS MARKET, BY DISEASE TYPE
8.1 OVERVIEW
8.2 TRAUMATIC
8.3 NON-TRAUMATIC
8.4 IDIOPATHIC
9 U.S. OSTEONECROSIS MARKET, BY TYPE
9.1 OVERVIEW
9.2 TREATMENT
9.2.1 SURGERY
9.2.1.1 CORE DECOMPRESSION
9.2.1.2 BONE RESHAPING (OSTEOTOMY)
9.2.1.3 BONE TRANSPLANT
9.2.1.4 JOINT REPLACEMENT THERAPY
9.2.1.5 STEM CELL THERAPY
9.2.2 MEDICATION
9.2.2.1 NSAIDS
9.2.2.1.1 IBUPROFEN
9.2.2.1.2 ASPIRIN
9.2.2.1.3 NAPROXEN
9.2.2.1.4 DICLOFENAC
9.2.2.1.5 OTHERS
9.2.2.2 BLOOD THINNERS
9.2.2.2.1 WARFARIN
9.2.2.2.2 HEPARIN
9.2.2.2.3 ENOXAPARIN
9.2.2.2.4 ARGATROBAN
9.2.2.2.5 DICOUMARAL
9.2.2.2.6 FONDAPARINUX
9.2.2.2.7 XIMELAGATRAN
9.2.2.3 OSTEOPOROSIS DRUGS
9.2.2.3.1 ALENDRONATE
9.2.2.3.2 RISEDRONATE
9.2.2.3.3 IBANDRONATE
9.2.2.3.4 ZOLEDRONIC ACID
9.2.2.3.5 DENOSUMAB
9.2.2.3.6 OTHERS
9.2.2.4 CHOLESTEROL-LOWERING DRUGS
9.2.2.4.1 ATORVASTATIN
9.2.2.4.2 PRAVASTATIN
9.2.2.4.3 FLUVASTATIN
9.2.2.4.4 ROSUVASTATIN
9.2.2.4.5 SIMVASTATIN
9.2.2.4.6 PITAVASTATIN
9.2.2.4.7 LOVASTATIN
9.2.2.5 OTHERS
9.2.3 PHYSICAL THERAPY
9.2.3.1 CRUTCHES
9.2.3.1.1 AXILLA CRUTCHES
9.2.3.1.1.1 METAL ALLOY
9.2.3.1.1.2 WOOD
9.2.3.1.1.3 CARBON OR GLASS FIBER REINFORCED COMPOSITE
9.2.3.1.1.4 OTHERS
9.2.3.1.2 ELBOW CRUTCHES
9.2.3.1.2.1 OPEN-CUFF ELBOW CRUTCHES
9.2.3.1.2.1.1 METAL ALLOY
9.2.3.1.2.1.2 WOOD
9.2.3.1.2.1.3 CARBON OR GLASS FIBER REINFORCED COMPOSITE
9.2.3.1.2.1.4 OTHERS
9.2.3.1.2.2 CLOSED-CUFF ELBOW CRUTCHES
9.2.3.1.2.2.1 METAL ALLOY
9.2.3.1.2.2.2 WOOD
9.2.3.1.2.2.3 CARBON OR GLASS FIBER REINFORCED COMPOSITE
9.2.3.1.2.2.4 OTHERS
9.2.3.1.3 GUTTER CRUTCHES
9.2.3.1.3.1 METAL ALLOY
9.2.3.1.3.2 WOOD
9.2.3.1.3.3 CARBON OR GLASS FIBER REINFORCED COMPOSITE
9.2.3.1.3.4 OTHERS
9.2.3.2 CASTING AND BRACING
9.2.3.2.1 NEWINGTON BRACE
9.2.3.2.2 TORONTO ORTHOSIS
9.2.3.2.3 SCOTTISH RITE ORTHOSIS
9.2.3.2.4 BROOMSTICK PLASTERS
9.2.3.2.5 BIRMINGHAM ORTHOSIS
9.2.3.3 OTHERS
9.2.3.3.1 CONVENTIONAL CALIPERS
9.2.3.3.2 SNYDER SLINGS
9.2.3.3.3 SLINGS WITH CRUTCHES
9.2.3.3.4 TRACTION
9.2.3.3.5 OTHERS
9.3 DIAGNOSIS
9.3.1 IMAGING TEST
9.3.1.1 MAGNETIC RESONANCE IMAGING (MRI)
9.3.1.1.1 CLOSED BORE
9.3.1.1.1.1 LOW FIELD STRENGTH
9.3.1.1.1.2 MID FIELD STRENGTH
9.3.1.1.1.3 HIGH FIELD STRENGTH
9.3.1.1.2 OPEN BORE
9.3.1.1.2.1 LOW FIELD STRENGTH
9.3.1.1.2.2 MID FIELD STRENGTH
9.3.1.1.2.3 HIGH FIELD STRENGTH
9.3.1.2 COMPUTED TOMOGRAPHY SCANNING
9.3.1.2.1 HIGH END SLICE
9.3.1.2.2 MID END SLICE
9.3.1.2.3 LOW END SLICE
9.3.1.2.4 CONE BEAN
9.3.1.3 X-RAY IMAGING
9.3.1.3.1 RADIOGRAPHY
9.3.1.3.1.1 DIGITAL IMAGING
9.3.1.3.1.2 FILM-BASED IMAGING
9.3.1.3.2 FLUOROSCOPY
9.3.1.3.2.1 DIGITAL IMAGING
9.3.1.3.2.2 FILM-BASED IMAGING
9.3.1.3.3 OTHERS
9.3.1.4 OTHERS
9.3.1.4.1 BONE SCAN
9.3.1.4.2 ULTRASOUND
9.3.1.4.2.1 2D ULTRASOUND
9.3.1.4.2.1.1 B/W ULTRASOUND
9.3.1.4.2.1.2 COLOURED
9.3.1.4.2.2 DOPPLER ULTRASOUND
9.3.1.4.2.2.1 B/W ULTRASOUND
9.3.1.4.2.2.2 COLOURED
9.3.1.4.2.3 3D & 4D ULTRASOUND
9.3.1.4.2.3.1 B/W ULTRASOUND
9.3.1.4.2.3.2 COLOURED
9.3.1.4.3 OTHERS
9.3.2 BIOPSY
9.3.2.1 NEEDLE BIOPSY
9.3.2.2 OPEN BIOPSY
9.3.2.3 OTHERS
9.3.3 OTHERS
10 U.S. OSTEONECROSIS MARKET, BY STAGES
10.1 OVERVIEW
10.2 STAGE 2
10.3 STAGE 3
10.4 STAGE 4
10.5 STAGE 1
11 U.S. OSTEONECROSIS MARKET, BY LOCATION
11.1 OVERVIEW
11.2 OSTEONECROSIS OF HIP/FEMORAL HEAD OSTEONECROSIS
11.2.1 TRAUMATIC
11.2.2 NON-TRAUMATIC
11.2.3 IDIOPATHIC OSTEONECROSIS
11.2.3.1 LEGG-CALVE-PERTHES DISEASE
11.2.3.1.1 TREATMENT
11.2.3.1.1.1 NON-SURGICAL
11.2.3.1.1.1.1 ANTI-INFLAMMATORY MEDICATION
11.2.3.1.1.1.1.1 IBUPROFEN
11.2.3.1.1.1.1.2 ASPIRIN
11.2.3.1.1.1.1.3 NAPROXEN SODIUM
11.2.3.1.1.1.1.4 NABUMETONE
11.2.3.1.1.1.1.5 OTHERS
11.2.3.1.1.1.2 BISPHOSPHONATES MEDICATIONS
11.2.3.1.1.1.2.1 ALENDRONATE
11.2.3.1.1.1.2.2 RISEDRONATE
11.2.3.1.1.1.2.3 IBANDRONATE
11.2.3.1.1.1.2.4 ZOLEDRONIC ACID
11.2.3.1.1.1.2.5 PAMIDRONATE
11.2.3.1.1.1.2.6 OTHERS
11.2.3.1.1.1.3 CRUTCHES
11.2.3.1.1.1.3.1 AXILLA CRUTCHES
11.2.3.1.1.1.3.2 ELBOW CRUTCHES
11.2.3.1.1.1.3.3 GUTTER CRUTCHES
11.2.3.1.1.1.4 CASTINGS & BRACINGS
11.2.3.1.1.1.4.1 NEWINGTON BRACE
11.2.3.1.1.1.4.2 TORONTO ORTHOSIS
11.2.3.1.1.1.4.3 SCOTTISH RITE ORTHOSIS
11.2.3.1.1.1.4.4 BROOMSTICK PLASTERS
11.2.3.1.1.1.4.5 BIRMINGHAM ORTHOSIS
11.2.3.1.1.2 SURGICAL
11.2.3.1.1.2.1 FEMORAL OSTEOTOMY
11.2.3.1.1.2.1.1 FEMORAL DEROTATION OSTEOTOMY
11.2.3.1.1.2.1.2 VARUS DEROTATION OSTEOTOMY
11.2.3.1.1.2.2 INNOMINATE OSTEOTOMY
11.2.3.1.1.2.2.1 SALTER (SINGLE INNOMINATE OSTEOTOMY)
11.2.3.1.1.2.2.2 SUTHERLAND (DOUBLE INNOMINATE OSTEOTOMY)
11.2.3.1.1.2.2.3 STEEL, TONNIS OR CARLOS (TRIPLE INNOMINATE OSTEOTOMY)
11.2.3.1.1.2.2.4 GANZ (PERIACETABULAR)
11.2.3.1.1.2.3 SHELF ARTHROPLASTY
11.2.3.1.1.2.4 OTHERS
11.2.3.1.2 DIAGNOSIS
11.2.3.1.2.1 MAGNETIC RESONANCE IMAGING (MRI)
11.2.3.1.2.1.1 CLOSED BORE
11.2.3.1.2.1.1.1 LOW FIELD STRENGTH
11.2.3.1.2.1.1.2 MID FIELD STRENGTH
11.2.3.1.2.1.1.3 HIGH FIELD STRENGTH
11.2.3.1.2.1.2 OPEN BORE
11.2.3.1.2.1.2.1 LOW FIELD STRENGTH
11.2.3.1.2.1.2.2 MID FIELD STRENGTH
11.2.3.1.2.1.2.3 HIGH FIELD STRENGTH
11.2.3.1.2.2 COMPUTED TOMOGRAPHY SCANNING
11.2.3.1.2.2.1 HIGH END SLICE
11.2.3.1.2.2.2 MID END SLICE
11.2.3.1.2.2.3 LOW END SLICE
11.2.3.1.2.2.4 CONE BEAN
11.2.3.1.2.3 X-RAY IMAGING
11.2.3.1.2.3.1 RADIOGRAPHY
11.2.3.1.2.3.1.1 DIGITAL IMAGING
11.2.3.1.2.3.1.2 FILM-BASED IMAGING
11.2.3.1.2.3.2 FLUOROSCOPY
11.2.3.1.2.3.2.1 DIGITAL IMAGING
11.2.3.1.2.3.2.2 FILM-BASED IMAGING
11.2.3.1.2.3.3 OTHERS
11.2.3.1.2.4 OTHERS
11.2.3.2 OTHERS
11.3 OSTEONECROSIS OF THE KNEE
11.3.1 TRAUMATIC
11.3.2 NON-TRAUMATIC
11.3.3 IDIOPATHIC OSTEONECROSIS
11.4 OSTEONECROSIS OF THE SHOULDER
11.4.1 TRAUMATIC
11.4.2 NON-TRAUMATIC
11.4.3 IDIOPATHIC OSTEONECROSIS
11.5 OSTEONECROSIS OF THE TALUS
11.5.1 TRAUMATIC
11.5.2 NON-TRAUMATIC
11.5.3 IDIOPATHIC OSTEONECROSIS
11.6 OSTEONECROSIS OF THE LUNATE
11.6.1 TRAUMATIC
11.6.2 NON-TRAUMATIC
11.6.3 IDIOPATHIC OSTEONECROSIS
11.7 OSTEONECROSIS OF THE SCAPHOID
11.7.1 TRAUMATIC
11.7.2 NON-TRAUMATIC
11.7.3 IDIOPATHIC OSTEONECROSIS
11.8 OTHERS
12 U.S. OSTEONECROSIS MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 BRANDED
12.2.1 ORAL
12.2.1.1 TABLETS
12.2.1.2 CAPSULES
12.2.2 PARENTERAL
12.2.3 OTHERS
12.3 GENERIC
12.3.1 ORAL
12.3.1.1 TABLETS
12.3.1.2 CAPSULES
12.3.2 PARENTERAL
12.3.3 OTHERS
13 U.S. OSTEONECROSIS MARKET, BY PRESCRIPTION
13.1 OVERVIEW
13.2 PRESCRIBED DRUGS
13.3 OVER THE COUNTER DRUGS
14 U.S. OSTEONECROSIS MARKET, BY GENDER
14.1 OVERVIEW
14.2 MALE
14.3 FEMALE
15 U.S. OSTEONECROSIS MARKET, BY AGE
15.1 OVERVIEW
15.2 ADULT
15.3 GERIATRIC
15.4 CHILDREN
16 U.S. OSTEONECROSIS MARKET, BY END USER
16.1 OVERVIEW
16.2 HOSPITALS
16.2.1 PUBLIC
16.2.2 PRIVATE
16.3 ORTHOPEDIC CLINICS
16.4 SPECIALTY CLINICS
16.5 AMBULATORY CENTERS
16.6 OTHERS
17 U.S. OSTEONECROSIS MARKET, BY DISTRIBUTION CHANNEL
17.1 OVERVIEW
17.2 RETAIL SALES
17.2.1 HOSPITAL PHARMACY
17.2.2 RETAIL PHARMACY
17.2.3 ONLINE PHARMACY
17.3 DIRECT TENDER
18 U.S. OSTEONECROSIS MARKET, COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: U.S.
19 SWOT ANALYSIS
20 U.S. OSTEONECROSIS MARKET, CPS
20.1 TEVA PHARMACEUTICALS USA, INC. (SUBSIDIARY OF TEVA PHARMACEUTICAL INDUSTRIES LTD.)
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 PRODUCT PORTFOLIO
20.1.4 RECENT DEVELOPMENT
20.2 PFIZER INC. (2022)
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 PRODUCT PORTFOLIO
20.2.4 RECENT DEVELOPMENT
20.3 BAYER AG (2022)
20.3.1 COMPANY SNAPSHOT
20.3.2 REVENUE ANALYSIS
20.3.3 PRODUCT PORTFOLIO
20.3.4 RECENT DEVELOPMENT
20.4 STRYKER
20.4.1 COMPANY SNAPSHOT
20.4.2 REVENUE ANALYSIS
20.4.3 PRODUCT PORTFOLIO
20.4.4 RECENT DEVELOPMENT
20.5 ARTHREX, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 PRODUCT PORTFOLIO
20.5.3 RECENT DEVELOPMENT
20.6 ALMATICA PHARMA
20.6.1 COMPANY SNAPSHOT
20.6.2 PRODUCT PORTFOLIO
20.6.3 RECENT DEVELOPMENT
20.7 DR. REDDY'S LABORATORIES LTD. (2022)
20.7.1 COMPANY SNAPSHOT
20.7.2 REVENUE ANALYSIS
20.7.3 PRODUCT PORTFOLIO
20.7.4 RECENT DEVELOPMENT
20.8 HALEON GROUP OF COMPANIES
20.8.1 COMPANY SNAPSHOT
20.8.2 REVENUE ANALYSIS
20.8.3 PRODUCT PORTFOLIO
20.8.4 RECENT DEVELOPMENT
20.9 SCIEGEN PHARMACEUTICALS.
20.9.1 COMPANY SNAPSHOT
20.9.2 PRODUCT PORTFOLIO
20.9.3 RECENT DEVELOPMENT
20.1 ZIMMER BIOMET
20.10.1 COMPANY SNAPSHOT
20.10.2 REVENUE ANALYSIS
20.10.3 PRODUCT PORTFOLIO
20.10.4 RECENT DEVELOPMENT
21 QUESTIONNAIRE
22 RELATED REPORTS
表格列表
TABLE 1 COST OF OSTEONECROSIS TREATMENT SURGERIES
TABLE 2 U.S. OSTEONECROSIS MARKET, PIPELINE ANALYSIS
TABLE 3 U.S. OSTEONECROSIS MARKET, BY DISEASE TYPE, 2021-2030 (USD THOUSAND)
TABLE 4 U.S. OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 5 U.S. TREATMENT IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 6 U.S. SURGERY IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 7 U.S. MEDICATION IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 8 U.S. NSAIDS IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 9 U.S. BLOOD THINNERS IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 10 U.S. OSTEOPOROSIS DRUGS IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 11 U.S. CHOLESTEROL-LOWERING DRUGS IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 12 U.S. PHYSICAL THERAPY IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 13 U.S. CRUTCHES IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 14 U.S. AXILLA CRUTCHES IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 15 U.S. ELBOW CRUTCHES IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 16 U.S. OPEN-CUFF ELBOW CRUTCHES IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 17 U.S. CLOSED-CUFF ELBOW CRUTCHES IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 18 U.S. GUTTER CRUTCHES IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 19 U.S. CASTING AND BRACING IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 20 U.S. OTHERS IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 21 U.S. DIAGNOSIS IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 22 U.S. IMAGING TEST IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 23 U.S. MAGNETIC RESONANCE IMAGING (MRI) IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 24 U.S. CLOSED BORE IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 25 U.S. OPEN BORE IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 26 U.S. COMPUTED TOMOGRAPHY SCANNING IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 27 U.S. X-RAY IMAGING IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 28 U.S. RADIOGRAPHY IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 29 U.S. FLUOROSCOPY IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 30 U.S. OTHERS IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 31 U.S. ULTRASOUND IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 32 U.S. 2D ULTRASOUND IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 33 U.S. DOPPLER ULTRASOUND IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 34 U.S. 3D & 4D ULTRASOUND IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 35 U.S. BIOPSY IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 36 U.S. OSTEONECROSIS MARKET, BY STAGES, 2021-2030 (USD THOUSAND)
TABLE 37 U.S. OSTEONECROSIS MARKET, BY LOCATION, 2021-2030 (USD THOUSAND)
TABLE 38 U.S. OSTEONECROSIS OF HIP/FEMORAL HEAD OSTEONECROSIS IN OSTEONECROSIS MARKET, BY DISEASE TYPE, 2021-2030 (USD THOUSAND)
TABLE 39 U.S. IDIOPATHIC OSTEONECROSIS IN OSTEONECROSIS MARKET, BY DISEASE TYPE, 2021-2030 (USD THOUSAND)
TABLE 40 U.S. LEGG-CALVE-PERTHES DISEASE IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 41 U.S. TREATMENT IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 42 U.S. NON-SURGICAL IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 43 U.S. ANTI-INFLAMMATORY MEDICATIONS IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 44 U.S. BISPHOSPHONATES MEDICATIONS IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 45 U.S. CRUTCHES IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 46 U.S. CASTING AND BRACING IN OSTEONECROSIS MARKET, BY TREATMENT, 2021-2030 (USD THOUSAND)
TABLE 47 U.S. SURGICAL IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 48 U.S. FEMORAL OSTEOTOMY IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 49 U.S. INNOMINATE OSTEOTOMY IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 50 U.S. DIAGNOSIS IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 51 U.S. MAGNETIC RESONANCE IMAGING (MRI) IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 52 U.S. CLOSED BORE IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 53 U.S. OPEN BORE IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 54 U.S. COMPUTED TOMOGRAPHY SCANNING IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 55 U.S. X-RAY IMAGING IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 56 U.S. RADIOGRAPHY IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 57 U.S. FLUOROSCOPY IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 58 U.S. OSTEONECROSIS OF THE KNEE IN OSTEONECROSIS MARKET, BY DISEASE TYPE, 2021-2030 (USD THOUSAND)
TABLE 59 U.S. OSTEONECROSIS OF THE SHOULDER IN OSTEONECROSIS MARKET, BY DISEASE TYPE, 2021-2030 (USD THOUSAND)
TABLE 60 U.S. OSTEONECROSIS OF THE TALUS IN OSTEONECROSIS MARKET, BY DISEASE TYPE, 2021-2030 (USD THOUSAND)
TABLE 61 U.S. OSTEONECROSIS OF THE LUNATE IN OSTEONECROSIS MARKET, BY DISEASE TYPE, 2021-2030 (USD THOUSAND)
TABLE 62 U.S. OSTEONECROSIS OF THE SCAPHOID IN OSTEONECROSIS MARKET, BY DISEASE TYPE, 2021-2030 (USD THOUSAND)
TABLE 63 U.S. OSTEONECROSIS MARKET, BY DRUG TYPE, 2021-2030 (USD THOUSAND)
TABLE 64 U.S. BRANDED IN OSTEONECROSIS MARKET, BY DRUG TYPE, 2021-2030 (USD THOUSAND)
TABLE 65 U.S. ORAL IN OSTEONECROSIS MARKET, BY DRUG TYPE, 2021-2030 (USD THOUSAND)
TABLE 66 U.S. GENERIC IN OSTEONECROSIS MARKET, BY DRUG TYPE, 2021-2030 (USD THOUSAND)
TABLE 67 U.S. ORAL IN OSTEONECROSIS MARKET, BY DRUG TYPE, 2021-2030 (USD THOUSAND)
TABLE 68 U.S. OSTEONECROSIS MARKET, BY PRESCRIPTION, 2021-2030 (USD THOUSAND)
TABLE 69 U.S. OSTEONECROSIS MARKET, BY GENDER, 2021-2030 (USD THOUSAND)
TABLE 70 U.S. OSTEONECROSIS MARKET, BY AGE, 2021-2030 (USD THOUSAND)
TABLE 71 U.S. OSTEONECROSIS MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 72 U.S. HOSPITALS IN OSTEONECROSIS MARKET, BY TYPE, 2021-2030 (USD THOUSAND)
TABLE 73 U.S. OSTEONECROSIS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 74 U.S. RETAIL SALES IN OSTEONECROSIS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
图片列表
FIGURE 1 U.S. OSTEONECROSIS MARKET: SEGMENTATION
FIGURE 2 U.S. OSTEONECROSIS MARKET: DATA TRIANGULATION
FIGURE 3 U.S. OSTEONECROSIS MARKET: DROC ANALYSIS
FIGURE 4 U.S. OSTEONECROSIS MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 U.S. OSTEONECROSIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 U.S. OSTEONECROSIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 U.S. OSTEONECROSIS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 U.S. OSTEONECROSIS MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 U.S. OSTEONECROSIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 U.S. OSTEONECROSIS MARKET: SEGMENTATION
FIGURE 11 INCREASING NUMBER OF TRAUMATIC INJURIES IS EXPECTED TO DRIVE THE GROWTH OF THE U.S. OSTEONECROSIS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE TRAUMATIC SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE U.S. OSTEONECROSIS MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINS, OPPORTUNITIES, AND CHALLENGES OF THE ASIA-PACIFIC KNEE CARTILAGE REPAIR MARKET
FIGURE 14 U.S. OSTEONECROSIS MARKET: BY DISEASE TYPE, 2022
FIGURE 15 U.S. OSTEONECROSIS MARKET: BY DISEASE TYPE, 2023-2030 (USD THOUSAND)
FIGURE 16 U.S. OSTEONECROSIS MARKET: BY DISEASE TYPE, CAGR (2023-2030)
FIGURE 17 U.S. OSTEONECROSIS MARKET: BY DISEASE TYPE, LIFELINE CURVE
FIGURE 18 U.S. OSTEONECROSIS MARKET: BY TYPE, 2022
FIGURE 19 U.S. OSTEONECROSIS MARKET: BY TYPE, 2023-2030 (USD THOUSAND)
FIGURE 20 U.S. OSTEONECROSIS MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 21 U.S. OSTEONECROSIS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 U.S. OSTEONECROSIS MARKET: BY STAGES, 2022
FIGURE 23 U.S. OSTEONECROSIS MARKET: BY STAGES, 2023-2030 (USD THOUSAND)
FIGURE 24 U.S. OSTEONECROSIS MARKET: BY STAGES, CAGR (2023-2030)
FIGURE 25 U.S. OSTEONECROSIS MARKET: BY STAGES, LIFELINE CURVE
FIGURE 26 U.S. OSTEONECROSIS MARKET: BY LOCATION, 2022
FIGURE 27 U.S. OSTEONECROSIS MARKET: BY LOCATION, 2023-2030 (USD THOUSAND)
FIGURE 28 U.S. OSTEONECROSIS MARKET: BY LOCATION, CAGR (2023-2030)
FIGURE 29 U.S. OSTEONECROSIS MARKET: BY LOCATION, LIFELINE CURVE
FIGURE 30 U.S. OSTEONECROSIS MARKET: BY DRUG TYPE, 2022
FIGURE 31 U.S. OSTEONECROSIS MARKET: BY DRUG TYPE, 2023-2030 (USD THOUSAND)
FIGURE 32 U.S. OSTEONECROSIS MARKET: BY DRUG TYPE, CAGR (2023-2030)
FIGURE 33 U.S. OSTEONECROSIS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 34 U.S. OSTEONECROSIS MARKET: BY PRESCRIPTION, 2022
FIGURE 35 U.S. OSTEONECROSIS MARKET: BY PRESCRIPTION, 2023-2030 (USD THOUSAND)
FIGURE 36 U.S. OSTEONECROSIS MARKET: BY PRESCRIPTION, CAGR (2023-2030)
FIGURE 37 U.S. OSTEONECROSIS MARKET: BY PRESCRIPTION, LIFELINE CURVE
FIGURE 38 U.S. OSTEONECROSIS MARKET: BY GENDER, 2022
FIGURE 39 U.S. OSTEONECROSIS MARKET: BY GENDER, 2023-2030 (USD THOUSAND)
FIGURE 40 U.S. OSTEONECROSIS MARKET: BY GENDER, CAGR (2023-2030)
FIGURE 41 U.S. OSTEONECROSIS MARKET: BY GENDER, LIFELINE CURVE
FIGURE 42 U.S. OSTEONECROSIS MARKET: BY AGE, 2022
FIGURE 43 U.S. OSTEONECROSIS MARKET: BY AGE, 2023-2030 (USD THOUSAND)
FIGURE 44 U.S. OSTEONECROSIS MARKET: BY AGE, CAGR (2023-2030)
FIGURE 45 U.S. OSTEONECROSIS MARKET: BY AGE, LIFELINE CURVE
FIGURE 46 U.S. OSTEONECROSIS MARKET: BY END USER, 2022
FIGURE 47 U.S. OSTEONECROSIS MARKET: BY END USER, 2023-2030 (USD THOUSAND)
FIGURE 48 U.S. OSTEONECROSIS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 49 U.S. OSTEONECROSIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 50 U.S. OSTEONECROSIS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 51 U.S. OSTEONECROSIS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD THOUSAND)
FIGURE 52 U.S. OSTEONECROSIS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 53 U.S. OSTEONECROSIS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 54 U.S. OSTEONECROSIS MARKET: COMPANY SHARE 2022 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。