北美综合征多重诊断市场 - 按产品和服务(试剂和消耗品、仪器、软件和配件及服务)、感染类型(病毒、细菌、寄生虫和真菌)、疾病(呼吸道感染、胃肠炎、性传播感染、败血症、脑膜炎等)、小组类型(呼吸道小组、胃肠道小组、性传播疾病小组、血液败血症小组、脑膜炎小组等)、最终用户(临床实验室、医院、制药和生物技术公司、研究机构等)行业趋势和预测到 2029 年。
市场定义和见解
综合多重诊断是一种先进的诊断测试,用于检测传染病,例如呼吸道感染、传染性胃肠炎、性传播感染、败血症和脑膜炎等其他类型的传染病。综合多重诊断还可以帮助临床医生或医院检测各种疾病的症状和体征。这让医疗保健提供者可以为患者提供正确的治疗,并提供更精确的结果和可以更快地执行的护理。
综合征多重检测用于同时诊断多种病原体。在综合征多重诊断中,使用各种类型的试剂和耗材以及仪器和配件,有助于保持准确性并提供快速诊断结果。这些多重检测可快速诊断某些感染,从而可以迅速做出临床管理决策。基于多重技术的检测称为检测面板。综合征检测中使用的面板旨在诊断与相同或相似综合征类型相关的多种疾病。这些面板有助于在护理时评估疾病的原因。胃肠道面板和呼吸道面板是综合征面板的类型。
综合多重检测采用先进的多重 PCR 技术,借助综合多重诊断中使用的多个检测组,可在一小时内提供准确、快速的诊断结果。新一代综合多重检测可以快速识别呼吸道标本、血液和脑脊液中的常见病原体类型。使用多重检测组可以缩短周转时间,减少其他不必要的实验室测试,加快诊断速度并进行有针对性的治疗。
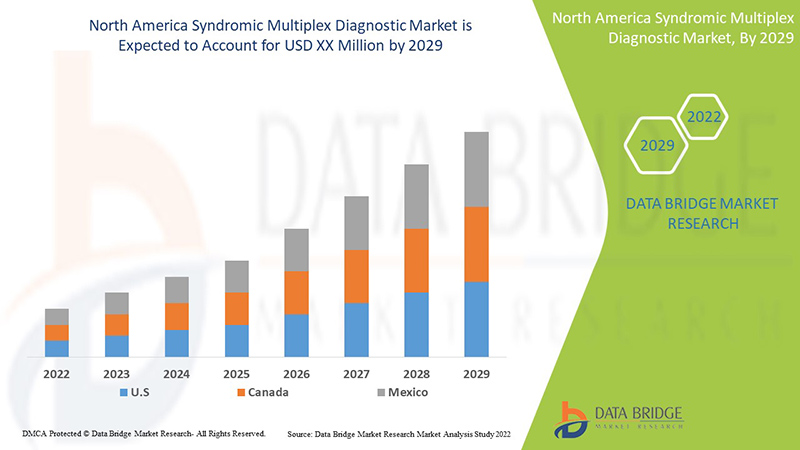
Data Bridge Market Research 分析,北美综合征多重诊断市场在 2022 年至 2029 年的预测期内将以 9.0% 的复合年增长率增长。
|
报告指标 |
细节 |
|
预测期 |
2022 至 2029 年 |
|
基准年 |
2021 |
|
历史岁月 |
2020 |
|
定量单位 |
收入(百万美元)、销量(单位)、定价(美元) |
|
涵盖的领域 |
产品和服务(试剂和耗材、仪器、软件和配件及服务)、感染类型(病毒、细菌、寄生虫和真菌)、疾病(呼吸道感染、胃肠炎、性传播感染、败血症、脑膜炎等)、检测组类型(呼吸道检测组、胃肠道-肠道检测组、性传播疾病检测组、血液-败血症检测组、脑膜炎检测组等)、最终用户(临床实验室、医院、制药和生物技术公司、研究机构等) |
|
覆盖国家 |
美国、加拿大和墨西哥 |
|
涵盖的市场参与者 |
BioFire Diagnostics(bioMérieux SA 的子公司)、Seegene Inc.(韩国)、Luminex Corporation. A DiaSorin Company(美国)、F. Hoffmann-La Roche Ltd(瑞士)、BD(美国)、Bio-Rad Laboratories, Inc.(美国)、Cepheid(Danaher(美国)的子公司)、QIAGEN(德国)、Abbott(美国)、Hologic, Inc.(美国)、Thermo Fisher Scientific Inc.(美国)、Siemens Healthcare GmbH(德国)、Akonni Biosystems, Inc.(美国)、Biocartis(比利时)、QuantuMDx Group Ltd.(英国)、Applied BioCode, Inc.(美国)、Prominex Inc.(美国)、Nanomix, Inc.(美国)、Curetis(OpGen, Inc. 的子公司)(德国) |
综合多重诊断市场动态
驱动程序
- 传染病发病率上升
传染性细菌和病毒性疾病发病率的上升影响了市场的需求,因为在综合征检测中,多重实时 PCR 技术和综合征方法用于传染病的分子诊断。
- 增加对急性呼吸道综合征冠状病毒检测的监管批准
2020 年 5 月,生物梅里埃公司获得了 FDA 对 BIOFIRE RP2.1 检测板的紧急使用授权 (EUA),该检测板用于检测引起呼吸道感染的 22 种病原体,包括用于检测 COVID-19 疾病的 SARS-CoV-2。
FDA 或 CE 标志等监管机构为商业化 SARS-CoV-2 检测面板和检测与 COVID-19 疾病相关的病毒提供了紧急使用授权 (EUA) 批准,这是推动市场增长的动力。
机会
- 市场参与者采取的战略举措
2021 年 3 月,F. Hoffman-La Roche Ltd 收购了领先的多重分子诊断提供商 GenMark Diagnostics。此次收购帮助该公司拓宽了罗氏的分子诊断产品组合。市场参与者采取的这些战略举措,包括有针对性的细分产品发布,正在帮助他们扩大全球影响力并增强其产品组合,并为市场的增长提供机会。
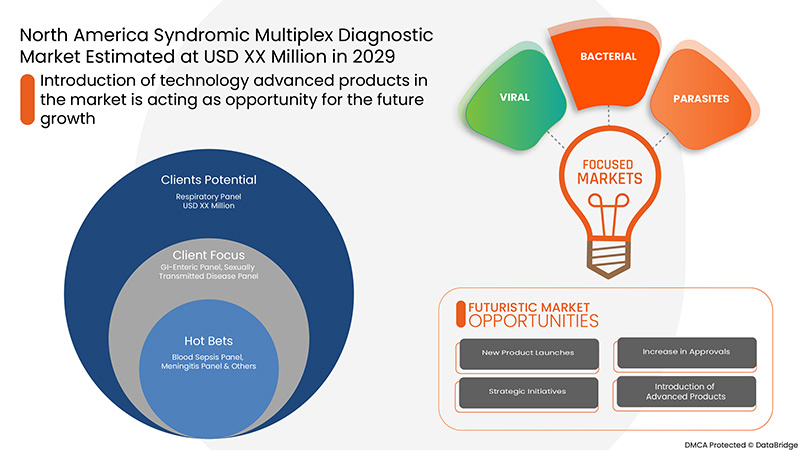
- 引进技术先进的产品
综合多重检测利用多重 PCR 的先进技术来检测、分离或扩增目标核酸,从而提供准确、快速的诊断结果;先进的技术可在 45 至 60 分钟内提供诊断结果。因此,技术先进的产品的开发是市场增长的机会。
克制/挑战
- 诊断产品成本高
多重综合征检测应用利用实时聚合酶链式反应(PCR),其结果具有扩增曲线和正确值。综合征多重诊断中使用的仪器需要高昂的维护成本。因此,仪器的高成本对市场是一个挑战。
COVID-19 对综合性多重诊断市场的影响
COVID-19 对市场产生了积极影响。市场参与者正在推出用于检测 SARS-CoV 病毒的不同产品。COVID-19 疫情过后,监管机构对产品的批准有所增加,从而推动了市场增长。
近期发展
- 2021 年 3 月,Luminex Corporation(Diasorin 旗下一家公司)获得了 FDA 紧急使用授权和 CE 标志,用于扩展 NxTAG 呼吸道面板测试。这项批准帮助该公司增加了收入。
综合多重诊断市场范围
综合多重诊断市场分为五个部分:产品和服务、感染类型、疾病、面板类型和最终用户。这些部分之间的增长将帮助您分析行业中增长微弱的部分,并为用户提供有价值的市场概览和市场洞察,帮助他们做出战略决策,以确定核心市场应用。
產品與服務
- 试剂和耗材
- 仪器、软件和配件
- 服务
根据产品和服务,北美综合征多重诊断市场细分为试剂和消耗品、仪器、软件和配件以及服务。
感染类型
- 病毒性的
- 细菌
- 寄生虫
- 真菌
根据感染类型,北美综合征多重诊断市场分为病毒、细菌、寄生虫和真菌。
疾病
- 呼吸道感染
- 胃肠道炎
- 性传播感染
- 脓毒症
- 脑膜炎
- 其他的
根据疾病,北美综合征多重诊断市场分为呼吸道感染、胃肠炎、性传播感染、败血症脑膜炎等。
面板类型
- 呼吸系统检查
- 胃肠道检查
- 性传播疾病小组
- 血液-败血症检查
- 脑膜炎小组
- 其他的
根据面板类型,北美综合征多重诊断市场细分为呼吸道面板、胃肠道面板、性传播疾病面板、血液败血症面板、脑膜炎面板和其他。
最终用户
- 医院
- 临床实验室
- 制药及生物技术公司
- 研究机构
- 其他的
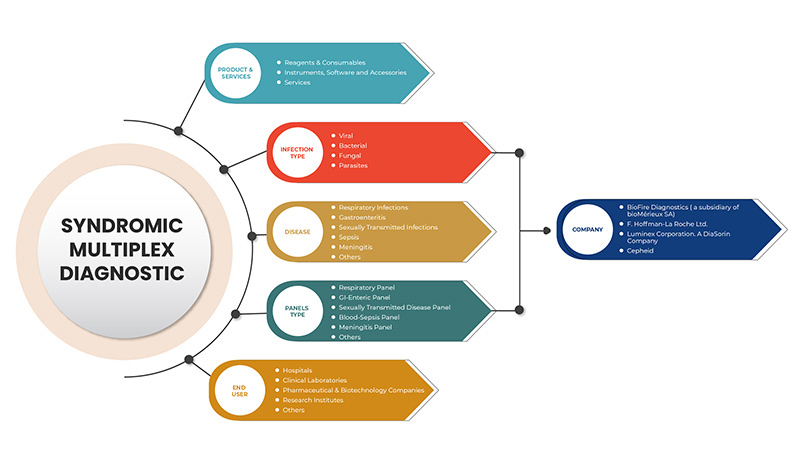
根据最终用户,北美综合多重诊断市场分为临床实验室、医院、制药和生物技术公司、研究机构和其他。
综合征多重诊断市场区域分析/见解
对综合征多重诊断市场进行了分析,并按国家、产品和服务、感染类型、疾病、面板类型和最终用户提供了市场规模洞察和趋势。
报告涉及的国家包括美国、加拿大和墨西哥。
由于传染病患病率的上升以及对早期和准确诊断的需求增加,美国综合征多重诊断市场预计将增长。
报告的国家部分还提供了影响单个市场因素和国内市场法规变化,这些变化影响了市场的当前和未来趋势。新销售、替代销售、国家人口统计、疾病流行病学和进出口关税等数据点是用于预测单个国家市场情景的一些主要指标。此外,在提供国家数据的预测分析时,还考虑了品牌的存在和可用性以及由于来自本地和国内品牌的激烈或稀缺竞争而面临的挑战、销售渠道的影响。
综合征多重诊断市场份额分析
综合多重诊断市场竞争格局按竞争对手提供详细信息。详细信息包括公司概况、公司财务状况、收入、市场潜力、研发投资、新市场计划、沙特阿拉伯业务、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度、应用主导地位。以上提供的数据点仅与公司对综合多重诊断市场的关注有关。
北美综合征多重诊断市场的一些主要参与者包括 BioFire Diagnostics(bioMérieux SA 的子公司)、Seegene Inc.、Luminex Corporation。DiaSorin Company、F. Hoffmann-La Roche Ltd、BD、Bio-Rad Laboratories, Inc.、Cepheid(Danaher 的子公司)、QIAGEN、Abbott、Hologic, Inc.、Thermo Fisher Scientific Inc.、Siemens Healthcare GmbH、Akonni Biosystems, Inc.、Biocartis、QuantuMDx Group Ltd.、Applied BioCode, Inc.、Prominex Inc.、Nanomix, Inc.、Curetis(OpGen, Inc. 的子公司)等。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT AND SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
4.3 MARKET SHARE PER PANEL, FOR TOP 3 PLAYERS (2021)
5 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF INFECTIOUS DISEASES
6.1.2 RISING ADOPTION OF MOLECULAR DIAGNOSTICS TECHNIQUES
6.1.3 INCREASING REGULATORY APPROVAL FOR SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-COV-2) TESTING
6.1.4 INCREASING DEMAND FOR FAST AND ACCURATE DIAGNOSTIC RESULTS
6.2 RESTRAINTS
6.2.1 HIGH COST OF DIAGNOSTIC PRODUCTS
6.2.2 EXPLICIT LIMITATION OF SYNDROMIC MULTIPLEX DIAGNOSTIC
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES TAKEN BY MARKET PLAYERS
6.3.2 RISING DIAGNOSTIC HEALTHCARE EXPENDITURE
6.3.3 INTRODUCTION OF TECHNOLOGICAL ADVANCED PRODUCTS
6.4 CHALLENGES
6.4.1 PRODUCT RECALLS
6.4.2 LACK OF SKILLED PROFESSIONALS AND BARRIERS FACED IN CONDUCTING DIAGNOSTIC TESTS
7 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT & SERVICES
7.1 OVERVIEW
7.2 REAGENTS & CONSUMABLES
7.3 INSTRUMENTS, SOFTWARE & ACCESSORIES
7.4 SERVICES
8 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE
8.1 OVERVIEW
8.2 VIRAL
8.2.1 CORONAVIRUS
8.2.2 INFLUENZA VIRUS
8.2.3 ADENOVIRUS
8.2.4 RHINOVIRUS
8.2.5 ROTAVIRUS
8.2.6 OTHERS
8.3 BACTERIAL
8.3.1 PNEUMONIAE
8.3.2 BORDETELLA PERTUSSIS
8.3.3 STAPHYLOCOCCUS
8.3.4 OTHERS
8.4 PARASITES
8.5 FUNGAL
9 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE
9.1 OVERVIEW
9.2 RESPIRATORY INFECTIONS
9.3 GASTROENTERITIS
9.4 SEXUALLY TRANSMITTED INFECTIONS
9.5 SEPSIS
9.6 MENINGITIS
9.7 OTHERS
10 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE
10.1 OVERVIEW
10.2 RESPIRATORY PANEL
10.3 GI-ENTERIC PANEL
10.4 SEXUALLY TRANSMITTED DISEASE PANEL
10.5 BLOOD-SEPSIS PANEL
10.6 MENINGITIS PANEL
10.7 OTHERS
11 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICAL LABORATORIES
11.4 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
11.5 RESEARCH INSTITUTES
11.6 OTHERS
12 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 BIOFIRE DIAGNOSTICS (A SUBSIDIARY OF BIOMÉRIEUX SA)
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 F. HOFFMANN-LA ROCHE LTD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 LUMINEX CORPORATION. A DIASORIN COMPANY
15.3.1 COMPANY SNAPSHOT
15.3.2 RECENT FINANCIALS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 CEPHEID (A SUBSIDIARY OF DANAHER)
15.4.1 COMPANY SNAPSHOT
15.4.2 RECENT FINANCIALS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 QIAGEN
15.5.1 COMPANY SNAPSHOT
15.5.2 RECENT FINANCIALS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 AKONNI BIOSYSTEMS, INC.
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 APPLIED BIOCODE, INC.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 BD
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 BIOCARTIS
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 BIO-RAD LABORATORIES, INC.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT
15.12 BOSCH HEALTHCARE SOLUTIONS GMBH (A SUBSIDIARY OF ROBERT BOSCH GMBH)
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 CURETIS (A SUBSIDIARY OF OPGEN, INC.)
15.13.1 COMPANY SNAPSHOT
15.13.2 RECENT FINANCIALS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 HOLOGIC, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 RECENT FINANCIALS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 MIRXES PTE LTD.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 NANŌMIX, INC.
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 PROMINEX INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 QUANTUMDX GROUP LTD.
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENTS
15.19 SEEGENE INC.
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 SIEMENS HEALTHCARE GMBH
15.20.1 COMPANY SNAPSHOT
15.20.2 RECENT FINANCIALS
15.20.3 PRODUCT PORTFOLIO
15.20.4 RECENT DEVELOPMENT
15.21 THERMOFISHER SCIENTIFIC INC.
15.21.1 COMPANY SNAPSHOT
15.21.2 RECENT FINANCIALS
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
表格列表
TABLE 1 COST OF THE PRODUCT
TABLE 2 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT & SERVICES, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA REAGENTS & CONSUMABLES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA INSTRUMENTS, SOFTWARE & ACCESSORIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA SERVICES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA PARASITES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA FUNGAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA RESPIRATORY INFECTIONS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA GASTROENTERITIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA SEXUALLY TRANSMITTED INFECTIONS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA SEPSIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA MENINGITIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA RESPIRATORY PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA GI-ENTERIC PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA SEXUALLY TRANSMITTED DISEASE PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA BLOOD-SEPSIS PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA MENINGITIS PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA HOSPITALS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA CLINICAL LABORATORIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA RESEARCH INSTITUTES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 41 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 42 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 43 U.S. VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 44 U.S. BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 45 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 46 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 47 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 49 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 50 CANADA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 51 CANADA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 52 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 53 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 54 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 56 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 57 MEXICO VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 58 MEXICO BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 59 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 60 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 61 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
图片列表
FIGURE 1 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE AND INCIDENCE OF INFECTIOUS DISEASES IS EXPECTED TO DRIVE THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET IN THE FORECAST PERIOD
FIGURE 12 REAGENTS & CONSUMABLES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET
FIGURE 15 TOTAL CASES OF COVID-19 IN NORTH AMERICA
FIGURE 16 TOTAL CASES OF COVID-19 IN EUROPE
FIGURE 17 NATIONAL HEALTH EXPENDITURE VS MEDICAL DEVICE EXPENDITURE, U.S., 2019
FIGURE 18 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, 2021
FIGURE 19 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, 2022-2029 (USD MILLION)
FIGURE 20 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, CAGR (2022-2029)
FIGURE 21 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, LIFELINE CURVE
FIGURE 22 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, 2021
FIGURE 23 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, 2022-2029 (USD MILLION)
FIGURE 24 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, CAGR (2022-2029)
FIGURE 25 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, 2021
FIGURE 27 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, 2022-2029 (USD MILLION)
FIGURE 28 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, CAGR (2022-2029)
FIGURE 29 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, 2021
FIGURE 31 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, 2022-2029 (USD MILLION)
FIGURE 32 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, CAGR (2022-2029)
FIGURE 33 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, LIFELINE CURVE
FIGURE 34 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, 2021
FIGURE 35 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 36 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, CAGR (2022-2029)
FIGURE 37 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SNAPSHOT (2021)
FIGURE 39 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2021)
FIGURE 40 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2029)
FIGURE 41 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2021 & 2029)
FIGURE 42 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT AND SERVICES (2022-2029)
FIGURE 43 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY SHARE 2021 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。