North America Multiple Sclerosis Treatment Market
市场规模(十亿美元)
CAGR :
% 
 USD
2,879.60 million
USD
5,737.78 million
2022
2030
USD
2,879.60 million
USD
5,737.78 million
2022
2030
| 2023 –2030 | |
| USD 2,879.60 million | |
| USD 5,737.78 million | |
|
|
|
北美多发性硬化症治疗市场、疾病类型(复发缓解型多发性硬化症 (RRMS)、继发性进行性多发性硬化症 (SPMS)、原发性进行性多发性硬化症 (PPMS)、严重复发缓解型多发性硬化症 (RES))、治疗(预防疗法、中止疗法/急性加重治疗、对症疗法)、药物类型(品牌药、仿制药)、最终用户(医院和诊所、诊断实验室、其他)– 行业趋势和预测到 2030 年。
北美多发性硬化症治疗市场分析和规模
众多传染病给全球医疗保健系统带来的负担不断增加,是市场增长的主要原因。与去年相比,随着多发性硬化症发病率的上升,对更好的多发性硬化症治疗方案的需求不断增加,政府举措也不断增加,多发性硬化症治疗需求也随之增加。发达国家和发展中国家的政府和非政府组织正在努力提高人们对多发性硬化症的认识,并为药物研究提供大量资金。
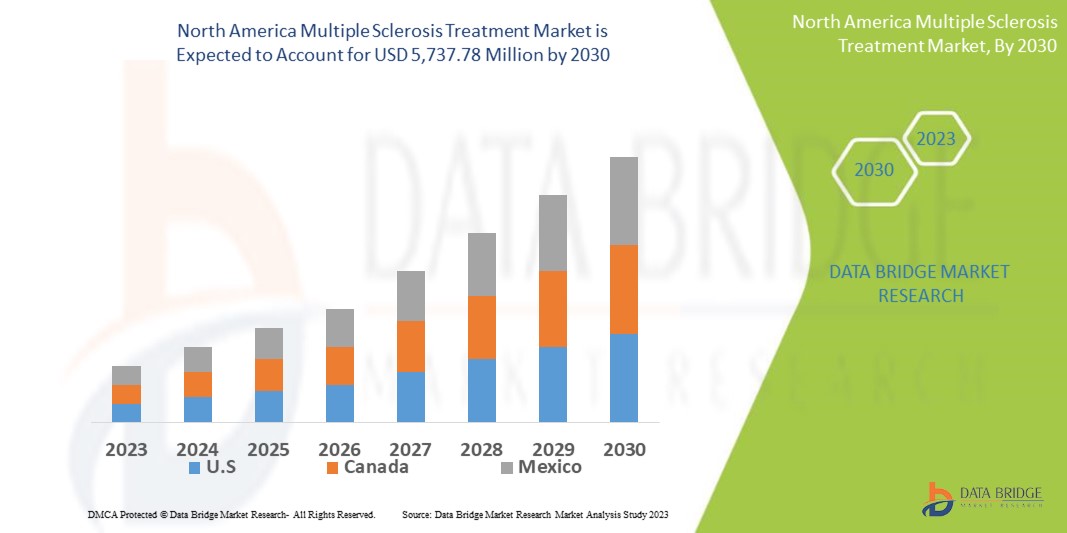
Data Bridge Market Research 分析,多发性硬化症治疗市场预计到 2030 年将达到 57.3778 亿美元,即 2022 年为 28.796 亿美元,预计在 2023 年至 2030 年的预测期内复合年增长率为 9%。除了对市场价值、增长率、细分、地理覆盖范围和主要参与者等市场情景的见解外,Data Bridge Market Research 策划的市场报告还包括深度专家分析、患者流行病学、管道分析、定价分析和监管框架。
北美多发性硬化症治疗市场范围和细分
|
报告指标 |
细节 |
|
预测期 |
2023 至 2030 年 |
|
基准年 |
2022 |
|
历史岁月 |
2021(可定制为 2015 - 2020) |
|
定量单位 |
收入(百万美元)、销量(单位)、定价(美元) |
|
涵盖的领域 |
疾病类型(复发缓解型多发性硬化症 (RRMS)、继发性进展型多发性硬化症 (SPMS)、原发性进展型多发性硬化症 (PPMS)、重度复发缓解型多发性硬化症 (RES))、治疗(预防性治疗、中止治疗/急性加重治疗、对症治疗)、药物类型(品牌药、仿制药)、最终用户(医院、创伤中心、门诊手术中心等) |
|
覆盖国家 |
北美的美国、加拿大和墨西哥 |
|
涵盖的市场参与者 |
AbbVie Inc.(美国)、Bausch Health Companies Inc.(加拿大)、Biora Therapeutics, Inc(美国)、Boehringer Ingelheim International Gmbh(德国)、Amgen Inc.(美国)、Pfizer Inc(美国)、F. Hoffmann-La Roche Ltd(瑞士)、Mylan NV(美国)、Novartis AG(瑞士)、Bayer AG(德国)、Bristol Myers Squibb Company(美国)、Biogen(美国)、Teva Pharmaceutical Industries Ltd(以色列)、Takeda Pharmaceutical Company Limited(日本)、Jazz Pharmaceuticals, Inc(英国)、Abbott(美国)、Bio-Rad Laboratories Inc.(美国)、Mylan NV(美国) |
|
市场机会 |
|
市场定义
多发性硬化症 (MS) 是一种慢性炎症性免疫介导疾病,可导致脊髓和脑神经细胞的轴突横断、脱髓鞘和神经退化。症状取决于受损神经的数量以及受影响神经的形式和位置。多发性硬化症会导致多种症状,包括疼痛、疲劳、视力丧失和协调性受损。这种疾病的持续时间、症状和严重程度因人而异。
北美多发性硬化症治疗市场动态
驱动程序
- 多个市场参与者增加药物开发流程
一些制药公司也在药物开发过程中投入巨资。他们计划针对不同的适应症来吸引大量客户。例如,为多发性硬化症开发的药物包括继发性进展型 MS、原发性进展型 MS、复发缓解型 MS 和髓鞘修复或神经保护。例如,诺华于 2021 年 3 月获得欧盟委员会批准,用于治疗复发型多发性硬化症 (RMS) 成人患者,这些患者具有临床或影像学特征定义的活动性疾病。因此,这一因素推动了市场增长。
- 品牌药物需求不断增长
品牌药物需求的不断增长促进了市场的增长。由于众多公司不时推出大量产品,仿制药难以压倒品牌药物,因此品牌药物的需求不断增长。此外,当提交新药申请并附有稳定性、功效、剂型、制造、安全性、标签、化学和包装证明时,FDA 会批准品牌药物,这大大增加了品牌药物的需求。因此,这将促进市场增长。
机会
- 增加产品发布
用于治疗多发性硬化症的药物不断增加,促进了市场增长。例如,Mylan NV 宣布推出其首款获得 FDA 批准的仿制药富马酸二甲酯缓释胶囊 120 毫克和 240 毫克,用于治疗复发型多发性硬化症 (MS)。新推出的药物在治疗上与 Biogen 的 Tecfidera 胶囊几乎相似。该公司在 COVID-19 大流行期间推出的这种获得 FDA 批准的新药增加了市场需求。
- 口服给药需求不断增长
最近批准用于治疗多发性硬化症的几种新型口服药物代表了治疗领域的重大进展。口服给药途径可提高患者满意度并提高治疗依从性。例如,欧盟委员会 (EC) 于 2021 年 6 月批准 Aubagio (特立氟胺) 用于治疗患有复发缓解型多发性硬化症 (RRMS) 的 10 至 17 岁儿科患者。Aubagio 是欧盟首个适用于儿童和成人 MS 一线治疗的口服多发性硬化症 (MS) 疗法。此外,百时美施贵宝公司于 2020 年 3 月获得美国 FDA 批准 ZEPOSIA (ozanimod) 0.92 毫克,用于治疗患有复发型多发性硬化症 (RMS) 的成人,以及复发缓解性疾病、临床孤立综合征和活动性继发性进展性疾病。因此,这一因素提高了市场增长率。
限制/挑战
- 多发性硬化症药物的副作用
多发性硬化症有多种不良反应,阻碍了市场的增长。流感样症状、胸痛、心率波动、罕见脑部感染和化疗样反应等影响阻碍了患者采用多发性硬化症药物。预计这一因素将限制市场的增长。
本多发性硬化症治疗市场报告详细介绍了最新发展、贸易法规、进出口分析、生产分析、价值链优化、市场份额、国内和本地市场参与者的影响,分析了新兴收入领域的机会、市场法规的变化、战略市场增长分析、市场规模、类别市场增长、应用领域和主导地位、产品批准、产品发布、地域扩展、市场技术创新。如需了解有关多发性硬化症治疗市场的更多信息,请联系 Data Bridge Market Research 获取分析师简报,我们的团队将帮助您做出明智的市场决策,实现市场增长。
最新动态:
- 2020年,Genzyme Corporation与Principia Biopharma Inc.签署了收购协议,此次收购的主要动机是为了加强其在多发性硬化症和其他免疫介导疾病领域的研究活动。
- 2020年,诺华宣布美国FDA批准Kesimpta以注射剂形式用于皮下使用,用于治疗成人复发型多发性硬化症(RMS),包括临床孤立综合征、复发缓解型和活动性继发进展型多发性硬化症。
北美多发性硬化症治疗市场范围
多发性硬化症治疗市场根据疾病类型、治疗、药物类型和最终用户进行细分。这些细分市场之间的增长将帮助您分析行业中增长微弱的细分市场,并为用户提供有价值的市场概览和市场洞察,帮助他们做出战略决策,确定核心市场应用。
疾病类型
- 复发缓解型多发性硬化症 (RRMS)
- 继发性进行性多发性硬化症 (SPMS)
- 原发性进行性多发性硬化症 (PPMS)
- 严重复发缓解型多发性硬化症 (RES)
治疗
- 预防疗法
- 急性加重的终止疗法/治疗
- 对症治疗
药物类型
- 品牌
- 通用的
给药途径
- 口服
- 肠外
终端用户
- 医院和诊所
- 诊断实验室
- 其他的
多发性硬化症治疗区域分析/见解
对多发性硬化症治疗市场进行了分析,并根据上述疾病类型、治疗、药物类型和最终用户提供了市场规模洞察和趋势。
多发性硬化症治疗市场报告涵盖的国家包括北美的美国、加拿大和墨西哥。
由于美国引入了最新的多发性硬化症治疗方案,预计美国将在 2023 年至 2030 年的预测期内引领市场。该地区药物研发的不断增加也促进了市场的增长。人们对多发性硬化症及其治疗的认识不断提高,有助于引领市场增长。
报告的国家部分还提供了影响单个市场因素和国内市场监管变化,这些因素和变化影响了市场的当前和未来趋势。下游和上游价值链分析、技术趋势和波特五力分析、案例研究等数据点是用于预测单个国家市场情景的一些指标。此外,在提供国家数据的预测分析时,还考虑了北美品牌的存在和可用性以及由于来自本地和国内品牌的激烈或稀缺竞争而面临的挑战、国内关税和贸易路线的影响。
竞争格局和北美多发性硬化症治疗市场份额分析
多发性硬化症治疗市场竞争格局提供了按竞争对手划分的详细信息。详细信息包括公司概况、公司财务状况、产生的收入、市场潜力、研发投资、新市场计划、北美业务、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度、应用主导地位。以上提供的数据点仅与公司对多发性硬化症治疗市场的关注有关。
多发性硬化症治疗市场的一些主要参与者包括:
- AbbVie Inc.(美国)
- Bausch Health Companies Inc.(加拿大)
- Biora Therapeutics, Inc(美国)
- 勃林格殷格翰国际有限公司 (德国)
- 安进公司 (美国)
- 辉瑞公司 (美国)
- F. Hoffmann-La Roche Ltd(瑞士)
- Mylan NV(美国)
- 诺华公司(瑞士)
- 拜耳公司(德国)
- 百时美施贵宝公司 (美国)
- Biogen(美国)
- 梯瓦制药工业有限公司 (以色列)
- 武田药品工业株式会社 (日本)
- Jazz Pharmaceuticals, Inc(英国)
- 雅培(美国)
- Bio-Rad Laboratories Inc.(美国)
- Mylan NV(美国)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。