North America Craniomaxillofacial Implants Market, By Type (Bone Graft Substitute, Mid-Face Implants, Cranial/Neuro Implants, Mandibular Orthognathic Implants, Distraction System, Cranial Flap Fixation Systems, Patient Specific Implants (PSI), Total Temporomandibular (TMJ) Replacement System, Dural Repair Products, Others), Material of Construction (Metal, Bone Graft Substitute, Polymers/Biomaterial, Others), Application Site (Internal Fixators, External Fixators), Surgery Type (Reconstructive Surgery, Trauma Surgeries, Plastic Surgeries, Orthognathic Surgeries, Dental Surgeries, Ent Surgeries, Others), Property Type (Non-Resorbable Fixators, Resorbable Fixators), End User (Hospitals, Speciality Clinics, Trauma Centres, Ambulatory Surgical Centers (Ascs), Others), Distribution Channel (Direct Tender, Retail Sales), Country (U.S., Canada, Mexico) Industry Trends & Forecast to 2029.
Market Analysis and Insights: North America Craniomaxillofacial Implants Market
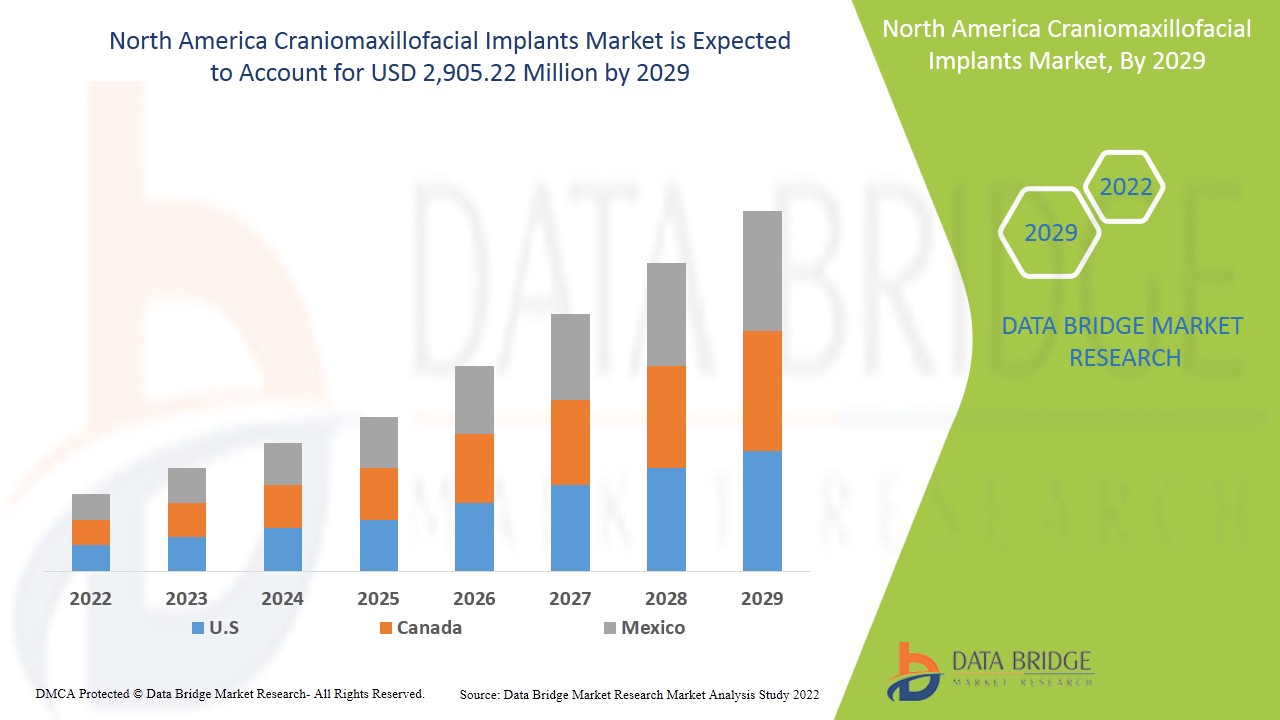
North America craniomaxillofacial implants market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 8.0% in the forecast period of 2022 to 2029 and is expected to reach USD 2,905.22 million by 2029. The increasing technological advancements is the major driver which propelled the demand of the market in the forecast period.
Craniomaxillofacial implants are employed for craniomaxillofacial surgeries. Craniomaxillofacial abnormalities occur during childbirth due to mother's folic acid deficiency or genetic mutations. Facial defects also damage speech, eating, language, and cause dental problems hearing loss. It is generally performed to treat injuries, defects, and diseases in the head, neck, face, jaws, hard and soft tissues of the oral and maxillofacial region.
The increase in geriatric population and rise in prevalence of bowel and bladder cancer are accelerating the growth of the market. The adaptation of advanced technologies in the industry has been stimulated the market growth consequently, the growing demand of patients specific implants in surgeries is likely to drive the market growth in the projected period.
The craniomaxillofacial implants market report provides details of market share, new developments, and product pipeline analysis, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario contact us for an analyst brief, our team will help you create a revenue impact solution to achieve your desired goal.
North America Craniomaxillofacial Implants Market Scope and Market Size
Craniomaxillofacial Implnats market is segmented on the basis of type, material of construction, application site, surgery type, property type, end user and distribution channel.
- On the basis of type, the craniomaxillofacial implants market is segmented into at bone graft substitute, mid-face implants, cranial/neuro implants, mandibular orthognathic implants, distraction system, cranial flap fixation systems, patient specific implants (PSI), total temporomandibular (TMJ) replacement system, dural repair products, and others. In 2022, bone graft substitute segment is expected to dominate the global craniomaxillofacial implants market due to increase in geriatric population globally.
- On the basis of material of construction, the craniomaxillofacial implants market is segmented into metal, bone graft substitute, polymers/biomaterial, and others. In 2022, metal is expected to dominate the global craniomaxillofacial implants market with the increasing technological advancement and healthcare expenditure government.
- On the basis of application site, the craniomaxillofacial implants market is segmented into external fixators and internal fixators. In 2022, external fixators is expected to dominate the global craniomaxillofacial implants market with the increasing product launches and acquisition.
- On the basis of surgery type, the craniomaxillofacial implants market is segmented into reconstructive surgery, trauma surgeries, plastic surgeries, orthognathic surgeries, dental surgeries, ENT surgeries, and others. In 2022, reconstructive surgeries is expected to dominate the global craniomaxillofacial implants market with the increasing number of surgeries and procedures.
- On the basis of property type, the craniomaxillofacial implants market is segmented resorbable fixators and non-resorbable fixators. In 2022, resorbable fixators is expected to dominate the global craniomaxillofacial implants market as they provide better result in lesser complication.
- On the basis of end user, the craniomaxillofacial implants market is segmented hospital, specialty clinics, trauma centers, ambulatory surgical centers (ASCS), and others. In 2022, hospital is expected to dominate the global craniomaxillofacial implants market with the increasing number of patients and surgeries.
- On the basis of distribution channel, the craniomaxillofacial implants market is segmented direct tender and retail sales. In 2022, direct tender is expected to dominate the global craniomaxillofacial implants market as they have all the major products from major market players.
Craniomaxillofacial Implants Market Country Level Analysis
Craniomaxillofacial Implnats market is segmented on the basis of type, material of construction, application site, surgery type, property type, end user and distribution channel.
The countries covered in the craniomaxillofacial implants market report are U.S., Canada, and Mexico.
- In North America, the U.S. is expected to dominate the market due to the presence of major market players.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Increasing Private Sector Funding and Technological Advancements are Boosting the Market Growth of Craniomaxillofacial Implants
Craniomaxillofacial implants market also provides you with detailed market analysis for every country growth in implants industry with impact of advancement, technology and changes in regulatory scenarios. The data is available for historic period 2011 to 2019.
Competitive Landscape and Craniomaxillofacial implants Market Share Analysis
Craniomaxillofacial implants market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to craniomaxillofacial implants market.
Major companies which are dealing in the craniomaxillofacial implants market are Zimmer Biomet, Stryker, Johnson and Johnson Services Inc., Medtronic, KLS Martin Group, Delphos Implants, Osteomed, Anatomics Pty Ltd, Bioplate Inc., Calavera Surgical Design, Innovasis, Integra LifeSciences Holdings Corp., Dimeda Instrumente GmbH, General Implants GmbH, Jeil Medical Corporation, Xilloc Medical BV, Ortho Select GmbH, B. Braun Melsungen AG, MONDEAL Medical Systems GmbH, OssDsign AB, TREU-Instrumente GmbH, Rebstock Instruments GmbH, 3D Systems, Inc., Medartis AG, BIOPORE SURGICALS, AlloSource, Teknimed, Poriferous, 7s Medical AG, Innovation Medical GmbH, Advin Health care, Gesco Healthcare Pvt. Ltd, Auxein Medical Private Limited Renishaw Plc, Ortho Max Manufacturing Company Pvt. Ltd. Vast Ortho, Skulle Implants Corporation., PANTHERA DENTAL, Lucid Implants among others.
- In October 2021, AlloSource announced that they are launching the AlloMend® Extra-Large (XL) Acellular Dermal Matrix (ADM), which is the newest addition to the AlloMend product line. This have increase companies product pipeline.
- On August 2021, Medtronic plc announced its agreement with Intersect ENT a global ear, nose, and throat (ENT) medical technology leader. Medtronic's acquisition of Intersect ENT expands the company's portfolio of products used during ear, nose, and throat procedures.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTERS FIVE FORCES MODEL
5 INDUSTRIAL INSIGHTS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE RATE OF CONGENITAL FACIAL DEFORMITIES
6.1.2 INCREASE IN TRAUMA CASES AND ROAD ACCIDENTS
6.1.3 INCREASING DEMAND FOR FACIAL RECONSTRUCTION SURGERY
6.1.4 RECENT TECHNOLOGICAL ADVANCEMENTS IN CMF IMPLANTS
6.2 RESTRAINS
6.2.1 HIGH COST OF SURGERIES AND IMPLANTS
6.2.2 IMPLANT MALFUNCTION AND ASSOCIATED RISK
6.2.3 LACK OF AWARENESS IN DEVELOPING COUNTRIES
6.3 OPPORTUNITIES
6.3.1 GROWING GERIATRIC POPULATION
6.3.2 RISE IN HEALTHCARE EXPENDITURE & INFRASTRUCTURE
6.3.3 GROWING R&D ACTIVITIES AND TECHNOLOGICAL ADVANCEMENT
6.3.4 INCREASE IN DEMAND FOR CUSTOMIZED AND PATIENT-SPECIFIC IMPLANTS
6.4 CHALLENGE
6.4.1 INCREASE IN PRODUCT RECALL
6.4.2 POST SURGERY COMPLIACTION
7 COVID-19 IMPACT ON NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET
7.1 PRICE IMPACT
7.2 IMPACT ON DEMAND
7.3 IMPACT ON SUPPLY
7.4 STRATEGIC DECISIONS BY MANUFACTURERS
7.5 CONCLUSION
8 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE
8.1 OVERVIEW
8.2 BONE GRAFT SUBSTITUTE
8.2.1 NATURAL
8.2.2 SYNTHETIC
8.3 MID-FACE IMPLANTS
8.3.1 PLATES
8.3.2 SCREWS
8.4 CRANIAL/NEURO IMPLANTS
8.4.1 PLATES
8.4.2 SCREWS
8.4.3 CONTOURABLE MESHES
8.5 MANDIBULAR ORTHOGNATHIC IMPLANTS
8.5.1 PLATES
8.5.2 SCREWS
8.6 DISTRACTION SYSTEM
8.7 CRANIAL FLAP FIXATION SYSTEMS
8.8 PATIENT SPECIFIC IMPLANTS (PSI)
8.8.1 METAL
8.8.2 PLASTIC
8.8.3 OTHERS
8.8.3.1 NORMAL
8.8.3.2 3D-PRINTED
8.8.3.2.1 CAD
8.8.3.2.2 ADEPT SOFTWARE
8.8.3.2.3 CAM
8.8.3.2.4 OTHERS
8.9 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM
8.1 DURAL REPAIR PRODUCTS
8.10.1 DURAL SEALANTS
8.10.2 DURAL SUBSTITUTES
8.11 OTHERS
9 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION
9.1 OVERVIEW
9.2 METALS
9.2.1 TITANIUM AND ALLOYS
9.2.2 STAINLESS STEEL
9.2.3 POLYETHERETHERKETONE (PEEK)
9.2.4 SILICON NITRIDE
9.2.5 OTHERS
9.3 BONE GRAFT SUBSTITUTE
9.4 POLYMERS/BIOMATERIAL
9.5 OTHERS
10 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE
10.1 OVERVIEW
10.2 INTERNAL FIXATOR
10.2.1 BONE GRAFT SUBSTITUTE
10.2.2 MID-FACE IMPLANTS
10.2.3 CRANIAL/NEURO IMPLANTS
10.2.4 MANDIBULAR ORTHOGNATHIC
10.2.5 DISTRACTION SYSTEM
10.2.6 CRANIAL FLAP FIXATION SYSTEMS
10.2.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
10.2.8 OTHERS
10.3 EXTERNAL FIXATOR
10.3.1 DISTRACTION SYSTEM
10.3.2 OTHERS
11 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE
11.1 OVERVIEW
11.2 RECONSTRUCTIVE SURGERY
11.2.1 BONE GRAFT SUBSTITUTE
11.2.2 MID-FACE IMPLANTS
11.2.3 CRANIAL/NEURO IMPLANTS
11.2.4 MANDIBULAR ORTHOGNATHIC
11.2.5 DISTRACTION SYSTEM
11.2.6 CRANIAL FLAP FIXATION SYSTEMS
11.2.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
11.2.8 DURAL REPAIR
11.2.9 OTHERS
11.3 TRAUMA SURGERY
11.3.1 BONE GRAFT SUBSTITUTE
11.3.2 MID-FACE IMPLANTS
11.3.3 CRANIAL/NEURO IMPLANTS
11.3.4 MANDIBULAR ORTHOGNATHIC
11.3.5 DISTRACTION SYSTEM
11.3.6 CRANIAL FLAP FIXATION SYSTEMS
11.3.7 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
11.3.8 DURAL REPAIR
11.3.9 OTHERS
11.4 PLASTIC SURGERIES
11.4.1 MID-FACE IMPLANTS
11.4.2 MANDIBULAR ORTHOGNATHIC
11.4.3 CRANIAL/NEURO IMPLANTS
11.4.4 DISTRACTION SYSTEM
11.4.5 CRANIAL FLAP FIXATION SYSTEMS
11.4.6 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
11.4.7 OTHERS
11.5 ORTHOGNATHIC SURGERIES
11.5.1 BONE GRAFT SUBSTITUTE
11.5.2 MANDIBULAR ORTHOGNATHIC
11.5.3 DISTRACTION SYSTEM
11.5.4 TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEMS
11.5.5 OTHERS
11.6 DENTAL SURGERIES
11.6.1 DISTRACTION SYSTEM
11.6.2 OTHERS
11.7 ENT SURGERIES
11.7.1 BONE GRAFT SUBSTITUTE
11.7.2 MID-FACE IMPLANTS
11.7.3 OTHERS
11.8 OTHERS
12 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE
12.1 OVERVIEW
12.2 NON-RESORBABLE FIXATORS
12.3 RESORBABLE FIXATORS
13 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITAL
13.3 SPECIALTY CLINICS
13.4 TRAUMA CENTERS
13.5 AMBULATORY SURGICAL CENTERS (ASCS)
13.6 OTHERS
14 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT TENDER
14.3 RETAIL SALES
15 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 ZIMMER BIOMET.
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 RODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENT
18.2 MEDTRONIC
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENT
18.3 DEPUY SYNTHES.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 STRYKER
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 ALLOSOURCE
18.5.1 COMPANY SNAPSHOT
18.5.2 COMPANY SHARE ANALYSIS
18.5.3 PRODUCT PORTFOLIO
18.5.4 RECENT DEVELOPMENT
18.5.4.1 FDA APPROVAL
18.6 ADVIN HEALTH CARE
18.6.1 COMPANY SNAPSHOT
18.6.2 PRODUCT PORTFOLIO
18.6.3 RECENT DEVELOPMENT
18.7 AESCULAP AG
18.7.1 COMPANY SNAPSHOT
18.7.2 PRODUCT PORTFOLIO
18.7.3 RECENT DEVELOPMENT
18.8 ANATOMICS PTY LTD.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 AUXEIN MEDICAL PRIVATE LIMITED
18.9.1 COMPANY SNAPSHOT
18.9.2 PRODUCT PORTFOLIO
18.9.3 RECENT DEVELOPMENT
18.1 BIOPLATE INC.
18.10.1 COMPANY SNAPSHOT
18.10.2 PRODUCT PORTFOLIO
18.10.3 RECENT DEVELOPMENT
18.11 BIOPORE SURGICALS
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENT
18.12 CALAVERA SURGICAL DESIGN
18.12.1 COMPANY SNAPSHOT
18.12.2 PRODUCT PORTFOLIO
18.12.3 RECENT DEVELOPMENT
18.13 DELPHOS IMPLANTS.
18.13.1 COMPANY SNAPSHOT
18.13.2 PRODUCT PORTFOLIO
18.13.3 RECENT DEVELOPMENT
18.14 DIMEDA INSTRUMENTE GMBH
18.14.1 COMPANY SNAPSHOT
18.14.2 PRODUCT PORTFOLIO
18.14.3 RECENT DEVELOPMENTS
18.15 GENERAL-IMPLANTS GMBH
18.15.1 COMPANY SNAPSHOT
18.15.2 PRODUCT PORTFOLIO
18.15.3 RECENT DEVELOPMENT
18.16 GESCO HEALTHCARE PVT. LTD.
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 INNOVATION MEDICAL GMBH
18.17.1 COMPANY SNAPSHOT
18.17.2 PRODUCT PORTFOLIO
18.17.3 RECENT DEVELOPMENT
18.17.3.1 MANUFACTURING CERTIFICATION
18.18 INNOVASIS INC.
18.18.1 COMPANY SNAPSHOT
18.18.2 PRODUCT PORTFOLIO
18.18.3 RECENT DEVELOPMENT
18.19 JEIL MEDICAL CORPORATION
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENT
18.2 KLS-MARTIN L.P.
18.20.1 COMPANY SNAPSHOT
18.20.2 PRODUCT PORTFOLIO
18.20.3 RECENT DEVELOPMENT
18.21 LUCID IMPLANTS
18.21.1 COMPANY SNAPSHOT
18.21.2 PRODUCT PORTFOLIO
18.21.3 RECENT DEVELOPMENT
18.22 MEDARTIS AG
18.22.1 COMPANY SNAPSHOT
18.22.2 PRODUCT PORTFOLIO
18.22.3 RECENT DEVELOPMENT
18.23 MONDEAL MEDICAL SYSTEMS GMBH
18.23.1 COMPANY SNAPSHOT
18.23.2 PRODUCT PORTFOLIO
18.23.3 RECENT DEVELOPMENT
18.24 ORTHO MAX MANUFACTURING COMPANY PVT. LTD.
18.24.1 COMPANY SNAPSHOT
18.24.2 PRODUCT PORTFOLIO
18.24.3 RECENT DEVELOPMENT
18.25 ORTHO SELECT GMBH
18.25.1 COMPANY SNAPSHOT
18.25.2 PRODUCT PORTFOLIO
18.25.3 RECENT DEVELOPMENT
18.26 OSTEOMED
18.26.1 COMPANY SNAPSHOT
18.26.2 PRODUCT PORTFOLIO
18.26.3 RECENT DEVELOPMENT
18.27 OSSDSIGN AB
18.27.1 COMPANY SNAPSHOT
18.27.2 PRODUCT PORTFOLIO
18.27.3 RECENT DEVELOPMENT
18.27.3.1 PRODUCT LAUNCH
18.27.3.2 ACQUISITION
18.28 PANTHERA DENTAL
18.28.1 COMPANY SNAPSHOT
18.28.2 PRODUCT PORTFOLIO
18.28.3 RECENT DEVELOPMENT
18.29 PORIFEROUS
18.29.1 COMPANY SNAPSHOT
18.29.2 PRODUCT PORTFOLIO
18.29.3 RECENT DEVELOPMENT
18.29.3.1 MANUFACTURING CERTIFICATION
18.3 REBSTOCK INSTRUMENTS GMBH
18.30.1 COMPANY SNAPSHOT
18.30.2 PRODUCT PORTFOLIO
18.30.3 RECENT DEVELOPMENT
18.31 RENISHAW PLC
18.31.1 COMPANY SNAPSHOT
18.31.2 REVENUE ANALYSIS
18.31.3 PRODUCT PORTFOLIO
18.31.4 RECENT DEVELOPMENT
18.32 SKULLE IMPLANTS CORPORATION.
18.32.1 COMPANY SNAPSHOT
18.32.2 PRODUCT PORTFOLIO
18.32.3 RECENT DEVELOPMENT
18.33 TEKNIMED
18.33.1 COMPANY SNAPSHOT
18.33.2 PRODUCT PORTFOLIO
18.33.3 RECENT DEVELOPMENT
18.33.3.1 MDSAP CERTIFICATION
18.34 TREU-INSTRUMENTE GMBH
18.34.1 COMPANY SNAPSHOT
18.34.2 PRODUCT PORTFOLIO
18.34.3 RECENT DEVELOPMENT
18.35 VAST ORTHO: ORTHOPEDIC IMPLANTS MANUFACTURERS
18.35.1 COMPANY SNAPSHOT
18.35.2 PRODUCT PORTFOLIO
18.35.3 RECENT DEVELOPMENTS
18.36 XILLOC MEDICAL BV
18.36.1 COMPANY SNAPSHOT
18.36.2 PRODUCT PORTFOLIO
18.36.3 RECENT DEVELOPMENT
18.37 7S MEDICAL AG
18.37.1 COMPANY SNAPSHOT
18.37.2 PRODUCT PORTFOLIO
18.37.3 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
表格列表
TABLE 1 UNITS/NUMBER OF PROCEDURES PERFORMED PER YEAR
TABLE 2 PRICING FOR VARIOUS PROCEDURES
TABLE 3 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 4 NORTH AMERICA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 5 NORTH AMERICA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 6 NORTH AMERICA MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 7 NORTH AMERICA MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 8 NORTH AMERICA CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 9 NORTH AMERICA CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 10 NORTH AMERICA MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 11 NORTH AMERICA MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 12 NORTH AMERICA DISTRACTION SYSTEM IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CRANIAL FLAP FIXATION SYSTEMS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 14 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 15 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 16 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 17 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 18 NORTH AMERICA TOTAL TEMPOROMANDIBULAR (TMJ) REPLACEMENT SYSTEM IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 19 NORTH AMERICA DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 20 NORTH AMERICA DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 21 NORTH AMERICA OTHERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 23 NORTH AMERICA METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 24 NORTH AMERICA METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 25 NORTH AMERICA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 26 NORTH AMERICA POLYMERS/BIOMATERIAL IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 27 NORTH AMERICA OTHERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 28 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 29 NORTH AMERICA INTERNAL FIXATOR IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 30 NORTH AMERICA INTERNAL FIXATOR IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 31 NORTH AMERICA EXTERNAL FIXATOR IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 32 NORTH AMERICA EXTERNAL FIXATOR IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 33 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 34 NORTH AMERICA RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 35 NORTH AMERICA RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 36 NORTH AMERICA TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 37 NORTH AMERICA TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 38 NORTH AMERICA PLASTIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 39 NORTH AMERICA PLASTIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 40 NORTH AMERICA ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 41 NORTH AMERICA ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 42 NORTH AMERICA DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 43 NORTH AMERICA DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 44 NORTH AMERICA ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 45 NORTH AMERICA ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 46 NORTH AMERICA OTHERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 47 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 48 NORTH AMERICA NON-RESORBABLE FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 49 NORTH AMERICA RESORBABLE FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 50 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 51 NORTH AMERICA HOSPITAL IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 52 NORTH AMERICA SPECIALTY CLINICS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 53 NORTH AMERICA TRAUMA CENTERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 54 NORTH AMERICA AMBULATORY SURGICAL CENTERS (ASCS) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 55 NORTH AMERICA OTHERS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 56 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
TABLE 57 NORTH AMERICA DIRECT TENDER IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 58 NORTH AMERICA RETAIL SALES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY REGION, 2015-2029 (USD MILLION)
TABLE 59 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY COUNTRY, 2015-2029 (USD MILLION)
TABLE 60 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 61 NORTH AMERICA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 62 NORTH AMERICA MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 63 NORTH AMERICA CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 64 NORTH AMERICA MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 65 NORTH AMERICA DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 66 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 67 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 68 NORTH AMERICA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 69 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 70 NORTH AMERICA METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 71 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 72 NORTH AMERICA INTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 73 NORTH AMERICA EXTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 74 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 75 NORTH AMERICA RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 76 NORTH AMERICA TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 77 NORTH AMERICA PLASTIC SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 78 NORTH AMERICA ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 79 NORTH AMERICA DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 80 NORTH AMERICA ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 81 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 82 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 83 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
TABLE 84 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 85 U.S. BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 86 U.S. MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 87 U.S. CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 88 U.S. MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 89 U.S. DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 90 U.S. PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 91 U.S. PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 92 U.S. PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 93 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 94 U.S. METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 95 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 96 U.S. INTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 97 U.S. EXTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 98 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 99 U.S. RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 100 U.S. TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 101 U.S. PLASTIC SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 102 U.S. ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 103 U.S. DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 104 U.S. ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 105 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 106 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 107 U.S. CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
TABLE 108 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 109 CANADA BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 110 CANADA MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 111 CANADA CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 112 CANADA MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 113 CANADA DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 114 CANADA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 115 CANADA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 116 CANADA PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 117 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 118 CANADA METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 119 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 120 CANADA INTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 121 CANADA EXTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 122 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 123 CANADA RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 124 CANADA TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 125 CANADA PLASTIC SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 126 CANADA ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 127 CANADA DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 128 CANADA ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 129 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 130 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 131 CANADA CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
TABLE 132 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 133 MEXICO BONE GRAFT SUBSTITUTE IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 134 MEXICO MID-FACE IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 135 MEXICO CRANIAL/NEURO IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 136 MEXICO MANDIBULAR ORTHOGNATHIC IMPLANTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 137 MEXICO DURAL REPAIR PRODUCTS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 138 MEXICO PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL, 2015-2029 (USD MILLION)
TABLE 139 MEXICO PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY TYPE, 2015-2029 (USD MILLION)
TABLE 140 MEXICO PATIENT SPECIFIC IMPLANTS (PSI) IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SOFTWARE, 2015-2029 (USD MILLION)
TABLE 141 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 142 MEXICO METALS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY MATERIAL OF CONSTRUCTION, 2015-2029 (USD MILLION)
TABLE 143 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 144 MEXICO INTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 145 MEXICO EXTERNAL FIXATORS IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY APPLICATION SITE, 2015-2029 (USD MILLION)
TABLE 146 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 147 MEXICO RECONSTRUCTIVE SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 148 MEXICO TRAUMA SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 149 MEXICO PLASTIC SURGERY IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 150 MEXICO ORTHOGNATHIC SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 151 MEXICO DENTAL SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 152 MEXICO ENT SURGERIES IN CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY SURGERY TYPE, 2015-2029 (USD MILLION)
TABLE 153 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY PROPERTY TYPE, 2015-2029 (USD MILLION)
TABLE 154 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY END USER, 2015-2029 (USD MILLION)
TABLE 155 MEXICO CRANIOMAXILLOFACIAL IMPLANTS MARKET, BY DISTRIBUTION CHANNEL, 2015-2029 (USD MILLION)
图片列表
FIGURE 1 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: NORTH AMERICA VS COUNTRY MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: SEGMENTATION
FIGURE 11 INCREASE IN DEMAND FOR PATIENTS SPECIFIC IMPLANTS ARE EXPECTED TO DRIVE THE NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 BONE GRAFT SUBSTITUTE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET IN 2022 & 2029
FIGURE 13 DRIVER, RESTRAINS, OPPORTUNITIES & CHALLENGES OF NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET
FIGURE 14 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE, 2021
FIGURE 15 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE, 2020-2029 (USD MILLION)
FIGURE 16 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 17 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 18 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY MATERIAL OF CONSTRUCTION, 2021
FIGURE 19 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY MATERIAL OF CONSTRUCTION, 2020-2029 (USD MILLION)
FIGURE 20 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY MATERIAL OF CONSTRUCTION, CAGR (2022-2029)
FIGURE 21 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY MATERIAL OF CONSTRUCTION, LIFELINE CURVE
FIGURE 22 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY APPLICATION SITE, 2021
FIGURE 23 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY APPLICATION SITE, 2020-2029 (USD MILLION)
FIGURE 24 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY APPLICATION SITE, CAGR (2022-2029)
FIGURE 25 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY APPLICATION SITE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY SURGERY TYPE, 2021
FIGURE 27 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY SURGERY TYPE, 2020-2029 (USD MILLION)
FIGURE 28 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY SURGERY TYPE, CAGR (2022-2029)
FIGURE 29 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY SURGERY TYPE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY PROPERTY TYPE, 2021
FIGURE 31 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY PROPERTY TYPE, 2020-2029 (USD MILLION)
FIGURE 32 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY PROPERTY TYPE, CAGR (2022-2029)
FIGURE 33 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY PROPERTY TYPE, LIFELINE CURVE
FIGURE 34 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY END USER, 2021
FIGURE 35 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 36 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY END USER, CAGR (2022-2029)
FIGURE 37 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 39 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 40 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 41 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 42 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: SNAPSHOT (2021)
FIGURE 43 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY COUNTRY (2021)
FIGURE 44 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 45 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 46 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: BY TYPE (2022-2029)
FIGURE 47 NORTH AMERICA CRANIOMAXILLOFACIAL IMPLANTS MARKET: COMPANY SHARE 2021 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。