Middle East And Africa Medical Device Regulatory Affairs Outsourcing Market
市场规模(十亿美元)
CAGR :
% 
 USD
2.06 Billion
USD
4.67 Billion
2025
2033
USD
2.06 Billion
USD
4.67 Billion
2025
2033
| 2026 –2033 | |
| USD 2.06 Billion | |
| USD 4.67 Billion | |
|
|
|
|
中东和非洲医疗器械监管事务外包市场,按服务(监管事务服务、质量咨询和医学写作)、产品(成品、电子产品和原材料)、设备类型(I 类、II 类和 III 类)、应用(心脏病学、诊断成像、骨科、IVD、眼科、普通和整形外科、药物输送、牙科、内窥镜检查、糖尿病护理和其他)、最终用户(小型医疗器械公司、中型医疗器械公司和大型医疗器械公司)、国家(沙特阿拉伯、南非、阿联酋、以色列、埃及和中东和非洲其他地区)行业趋势和预测到 2028 年
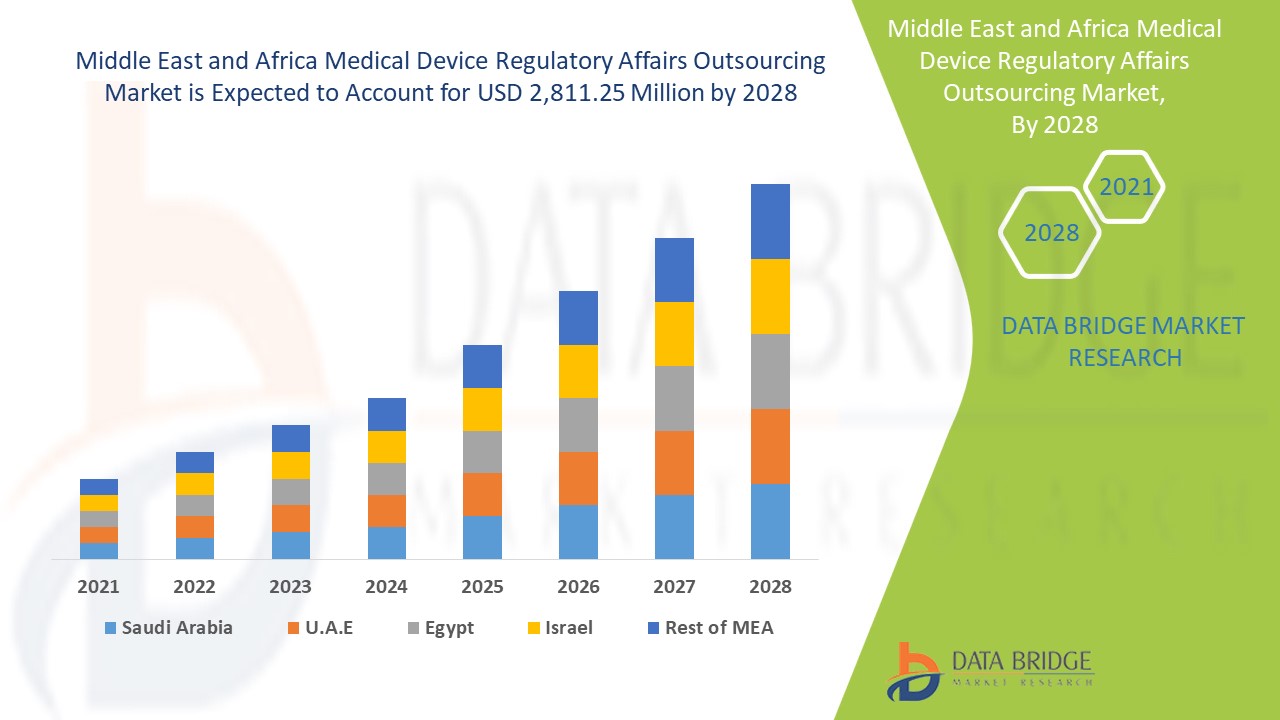 市场分析与洞察:中东和非洲医疗器械监管事务外包市场
市场分析与洞察:中东和非洲医疗器械监管事务外包市场
医疗器械监管事务外包市场预计将在 2021 年至 2028 年的预测期内实现市场增长。Data Bridge Market Research 分析称,在 2021 年至 2028 年的预测期内,该市场以 10.8% 的复合年增长率增长,预计到 2028 年将达到 28.1125 亿美元。地域扩张的战略举措预计将推动医疗器械监管事务外包市场的增长
外包是每家制药和生物技术公司在研发过程中价值链的重要组成部分。监管事务外包服务包括由专业医学作者、质量控制 (QC) 审计员和出版商撰写和发布监管文件,以促进高质量的临床研究项目。新兴经济体临床研究的大幅增加推动了对监管服务外包的需求,为该行业的增长提供了健康的平台。
专利到期数量的增加是医疗器械监管事务外包市场增长的驱动力。各种医疗器械监管事务服务价格的波动是医疗器械监管事务外包市场增长的制约因素。奖项和认可为医疗器械监管事务外包市场的增长提供了绝佳的机会。COVID-19 疫情的爆发对医疗器械监管事务外包市场的增长构成了挑战。
医疗器械监管事务外包市场报告提供了市场份额、新发展和产品线分析、国内和本地市场参与者的影响的详细信息,分析了新兴收入领域、市场法规变化、产品审批、战略决策、产品发布、地域扩张和市场技术创新方面的机会。要了解分析和医疗器械监管事务外包市场情况,请联系 Data Bridge Market Research 获取分析师简报,我们的团队将帮助您创建收入影响解决方案以实现您的预期目标。
 医疗器械监管事务外包市场范围和市场规模
医疗器械监管事务外包市场范围和市场规模
医疗器械监管事务外包市场根据服务、产品、设备类型、应用和最终用户进行细分。细分市场之间的增长有助于您分析利基增长领域和进入市场的策略,并确定您的核心应用领域和目标市场的差异。
- 根据服务,医疗器械法规事务外包市场细分为法规事务服务、质量咨询和医学写作。2021 年,由于主要医疗器械公司越来越多地采用法规事务外包,预计法规事务服务部门将主导医疗器械法规事务外包市场。
- 根据产品,医疗器械监管事务外包市场分为成品、电子产品和原材料。由于主要医疗器械公司越来越多地采用成品监管事务外包,预计 2021 年成品部分将主导医疗器械监管事务外包市场。
- 根据设备类型,医疗器械监管事务外包市场分为 I 类、II 类和 III 类。由于全球对治疗慢性病患者的医疗器械的需求不断增加,预计 2021 年 I 类细分市场将主导医疗器械监管事务外包市场。
- 根据应用,医疗器械监管事务外包市场细分为心脏病学、诊断成像、骨科、IVD、眼科、普通和整形外科、药物输送、牙科、内窥镜检查、糖尿病护理等。2021 年,心脏病学领域预计将在医疗器械监管事务外包市场中占据主导地位,因为主要医疗器械公司越来越多地采用 III 类医疗器械的监管事务外包。
- 根据最终用户,医疗器械监管事务外包市场分为小型医疗器械公司、中型医疗器械公司和大型医疗器械公司。由于全球对医疗器械的需求不断增长,预计 2021 年中型医疗器械公司将主导医疗器械监管事务外包市场。
医疗器械监管事务外包市场国家级分析
对医疗器械监管事务外包市场进行了分析,并按上述国家、服务、产品、设备类型、应用和最终用户提供了市场规模信息。
医疗器械监管事务外包市场报告涵盖的国家包括沙特阿拉伯、南非、阿联酋、以色列、埃及和中东及非洲其他地区。
由于监管事务服务部门的研发活动不断增加,沙特阿拉伯成为中东和非洲医疗器械监管事务外包市场增长领先的国家。
报告的国家部分还提供了影响单个市场因素和国内市场监管变化,这些因素和变化会影响市场的当前和未来趋势。新销售、替代销售、国家人口统计、监管法案和进出口关税等数据点是用于预测单个国家市场情景的一些主要指标。此外,在提供国家数据的预测分析时,还考虑了中东和非洲品牌的存在和可用性以及它们因来自本地和国内品牌的激烈或稀少竞争而面临的挑战、销售渠道的影响。
医疗器械公司不断增加的地域扩张活动正在推动医疗器械监管事务外包市场的增长
医疗器械监管事务外包市场还为您提供每个国家医疗器械监管事务外包行业增长的详细市场分析。此外,它还提供有关医疗器械监管事务外包销售、监管情景影响和医疗器械监管事务外包市场趋势参数的详细信息。数据适用于 2010 年至 2019 年的历史时期。
竞争格局和医疗器械监管事务外包市场份额分析
医疗器械监管事务外包市场竞争格局按竞争对手提供详细信息。详细信息包括公司概况、公司财务状况、产生的收入、市场潜力、研发投资、新市场计划、生产基地和设施、公司优势和劣势、产品发布、产品试验渠道、产品批准、专利、产品宽度和广度、应用优势、技术生命线曲线。以上提供的数据点仅与公司对医疗器械监管事务外包市场的关注有关。
报告中涉及的主要参与者包括 Parexel International Corporation、SGS SA、Intertek Group plc、WuXi AppTec、Charles River Laboratories、Celestica Inc.、Freyr、Eurofins Scientific、TÜV SÜD、Sterigenics US, LLC – A Sotera Health company、TE Connectivity、FLEX LTD.、Heraeus Holding、Integer Holdings Corporation、Nortech Systems, Inc.、IQVIA、Covance、Plexus Corp.、Sanmina Corporation、OMICS International、Omron Corporation 以及其他国内和全球参与者。DBMR 分析师了解竞争优势并为每个竞争对手分别提供竞争分析。
全球各地的公司也发起了许多合同和协议,这也加速了医疗器械监管事务外包市场的发展。
例如,
- 2020 年 1 月,Charles River Laboratories 宣布收购专门从事细胞治疗的公司 HemaCare Corporation(HemaCare)。该公司的战略性收购扩大了其早期研究和制造支持解决方案的产品组合,从而增加了销售额和收入。
市场参与者的合作、产品发布、业务扩展、奖励和认可、合资企业等策略正在增强公司在医疗器械监管事务外包市场的影响力,这也为组织的利润增长带来了好处。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。














