Global Wilsons Disease Drugs Market
市场规模(十亿美元)
CAGR :
% 
 USD
527.00 Million
USD
885.37 Million
2022
2030
USD
527.00 Million
USD
885.37 Million
2022
2030
| 2023 –2030 | |
| USD 527.00 Million | |
| USD 885.37 Million | |
|
|
|
|
Global Wilson’s Disease Drugs Market, By Tests Type (Blood and Urine Test, Eye Exam, Biopsy, Genetic Testing), Treatment (Medication, Surgery), Route of Administration (Oral, Parenteral, Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, online Pharmacies and Others) – Industry Trends and Forecast to 2030.
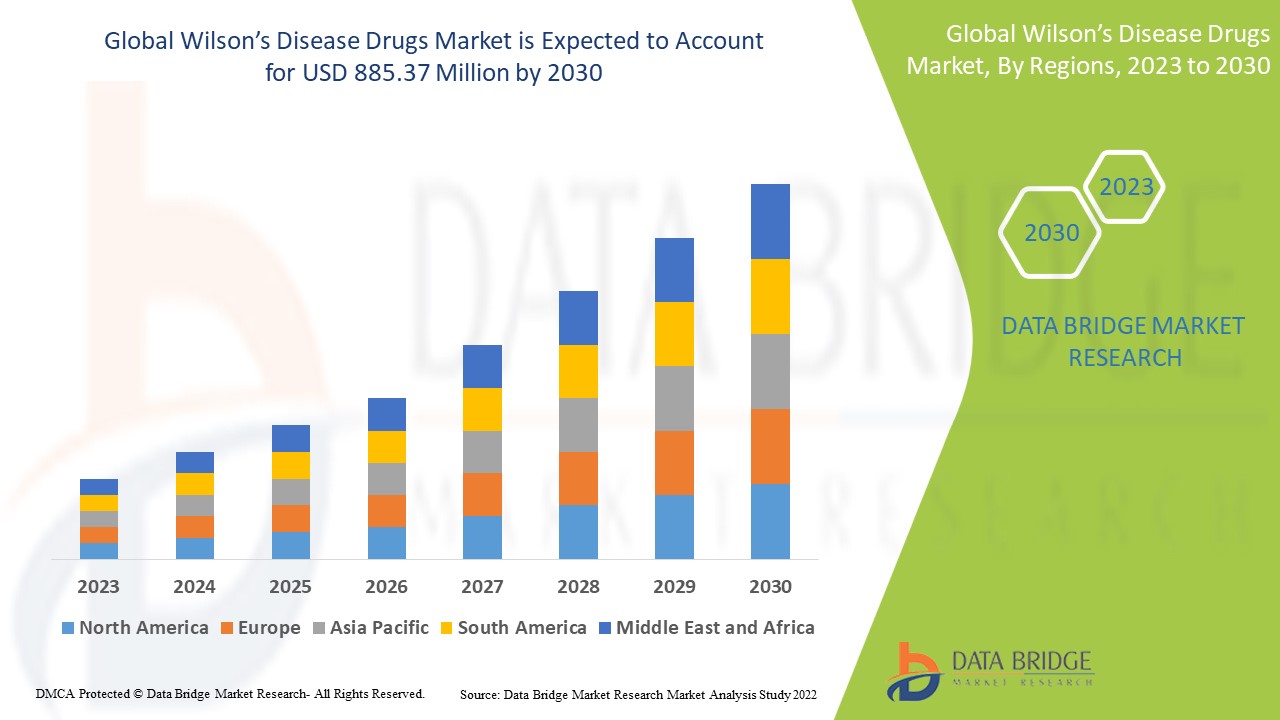
Wilson’s Disease Drugs Market Analysis and Size
The Wilson’s disease drugs market is expected to witness significant growth during the forecast period. As per the data published by National Library of Medicines, Wilson’s disease symptoms generally appear between the ages of 12 and 23 years, and untreated patients can live for up to 40 years. On the other hand, early detection and treatment of Wilson’s disease may help people live longer. Copper isn't adequately eliminated in Wilson’s illness, it accumulates in the body above normal levels.
Data Bridge Market Research analyses a growth rate in the Wilson’s disease drugs market in the forecast period 2023-2030. The expected CAGR of Wilson’s disease drugs market is tend to be around 6.7% in the mentioned forecast period. The market was valued at USD 527 million in 2022, and it would grow upto USD 885.37 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Wilson’s Disease Drugs Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Tests Type (Blood and Urine Test, Eye Exam, Biopsy, Genetic Testing), Treatment (Medication, Surgery), Route of Administration (Oral, Parenteral, Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, online Pharmacies and Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Amneal Pharmaceuticals LLC. (U.S.), Meda Pharmaceuticals (U.S.), Teva Pharmaceuticals Industries Ltd. (Israel), Taj Pharmaceutical Limited (India), Ipsen Pharma (France), TSUMURA & CO (Japan) |
|
Market Opportunities |
|
Market Definition
Wilson’s disease is a rare hereditary condition that causes copper accumulation in the liver, brain and other vital organs. Majority of people having Wilson’s disease are diagnosed between 5 and 35 years of age but it can also affect younger and older people. The symptoms for Wilson’s disease include abdominal pain, fatigue, jaundice, lack of appetite, problems with speech, swallowing or physical coordination, and uncontrolled movements or muscle stiffness.
Global Wilson’s Disease Drugs Market Dynamics
Drivers
- Increasing Technological Advancements
Growing advancement in techniques that are used for the diagnosis of Wilson’s disease and it’s treatment has projected to drive the market growth during the forecast period. For instance, according to an article published in Translational Gastroenterology and Hepatology Journal in April 2021, numerous advances in diagnosis and genetic testing have proved to be helpful in early diagnosis to prevent long-term complications of Wilson’s disease. Several individuals with Wilson’s disease can be diagnosed using the clinical findings of Kayser Fleischer (KF) rings, cirrhosis, neurological, or mental problems such as anxiety and depression in various combinations. Thus, it acts as a major driver in the market growth.
- Increase in the number of research and development activities
The market growth is fuelled by the increase in R&D activities. For instance, according to AstraZeneca websites, in August 2021 the FoCus Phase III trial in Wilson disease had reported positive high-level results in showing that ALXN1840 met the primary endpoint with a statistically notable advancement in daily mean copper mobilization from tissues, indicating superiority compared with standard-of-care (SoC) treatments. Focus is a crucial, Phase III, rater-blinded randomized, multi-center clinical trial manufactured to assess the safety and efficacy of ALXN1840 versus SoC in Wilson disease aged 12 years and older.
Opportunities
- Growing Awareness About the Disease
Numerous organizations are increasing awareness associated with Wilson’s disease, that is expected to expand the market growth during the forecast period. Wilson’s disease association, in New York, U.S., is a volunteer organization determined to promote the well-being of patients with Wilson’s disease. They offer the latest information about the disease and its symptoms and treatment. Furthermore, Wilson’s disease association supports research and clinical investigation for Wilson’s disease.
- Increasing Incidence of Wilson’s Disease
Increasing incidence of Wilson’s disease is because of the increase in the population globally, as Wilson’s disease is a rare genetic disorder which, is projected to favor growth for Wilson’s disease drugs market around the globe. For instance, in May 2022, according to data published by National Library of Medicine, National Institute of Health, high prevalence of Wilson’s disease is because of the increased rate of consanguineous marriages. Both males and females are equally affected by wilson’ disease.
Restraints/Challenges
- Lack of Diagnostic Facilities
Lack of several diagnostic facilities in hospitals and clinics is expected to limit the market growth. For instance, according to data published by National Center for Biotechnology Information (NCBI) in April 2019, no single examination can unequivocally confirm or exclude the disease. Diagnosis of patients with Wilson’s disease is challenging as it is based on a complex set of clinical findings derived from physical examination, patient history, imaging testing, and pathology laboratory with adequate facilities. Thus, lack of available facilities can restrain the market growth.
- Unavailability of Appropriate Treatments
To treat rare conditions, treatments are sometimes unavailable, particularly in underdeveloped countries. The severe patients need to be treated with advanced techniques, but these are sometimes unavailable in hospitals and clinics. Thus, it hampers the market growth.
This Wilson’s disease drugs market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Wilson’s disease drugs market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Wilson’s Disease Drugs Market Scope
The Wilson’s disease drugs market is segmented on the basis of test type, treatment, route of administration, end-user, distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Test Type
- Blood and Urine Test
- Eye Exam
- Biopsy
- Genetic Testing
Treatment
- Medication
- D-Penicillamine
- Trientine
- Zinc
- Tetrathiomolybdate
- Surgery
Route of Administration
- Oral
- Parenteral
- Others
End-Users
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacies
- Others
Wilson’s Disease Drugs Market Regional Analysis/Insights
The Wilson’s disease drugs market is analyzed and market size insights and trends are provided by test type, treatment, route of administration, end-user, and distribution channel as referenced above.
The major countries covered in the Wilson’s disease drugs market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is dominating the market due to the increasing medical tourism in developing countries and growing investment by market players.
Asia-Pacific is considered to have the most lucrative period due to the developing healthcare facilities, large number of generic manufacturers and rise in government initiatives.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Wilson’s Disease Drugs Market Share Analysis
The Wilson’s disease drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to Wilson’s disease drugs market.
Key players operating in the Wilson’s disease drugs market include:
- Amneal Pharmaceuticals LLC. (U.S.)
- Meda Pharmaceuticals (U.S.)
- Teva Pharmaceuticals Industries Ltd. (Israel)
- Taj Pharmaceutical Limited (India)
- Ipsen Pharma (France)
- TSUMURA & CO. (Japan)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。













