Global In Vitro Colorectal Cancer Screening Tests Market
市场规模(十亿美元)
CAGR :
% 
 USD
943.76 Million
USD
1,633.70 Million
2022
2030
USD
943.76 Million
USD
1,633.70 Million
2022
2030
| 2023 –2030 | |
| USD 943.76 Million | |
| USD 1,633.70 Million | |
|
|
|
|
Global In-Vitro Colorectal Cancer Screening Tests Market, By Product (Fecal Occult Blood Test, Biomarker Tests, and CRC DNA Screening Tests), End-User (Hospitals, Clinics, Diagnostics laboratories, and Ambulatory Surgical Centers), Imaging Type (Colonoscopy, Proctoscopy, CT Scan, Ultrasound, MRI, PET Scan), Application (MarCarePlex, Cologic, Colox, miRDIGN, PanCDx, and MeSorce CRC) – Industry Trends and Forecast to 2030.
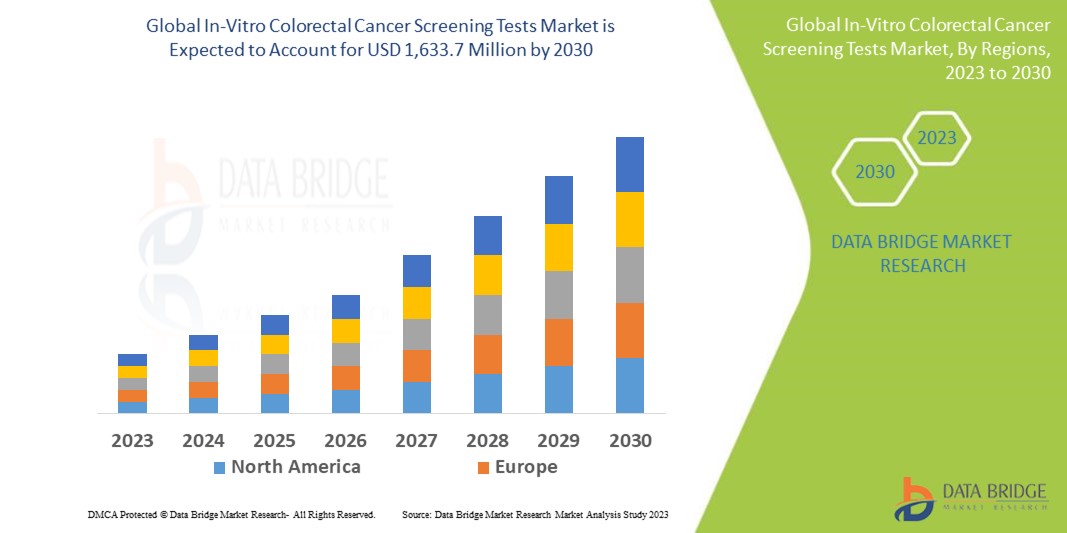
In-Vitro Colorectal Cancer Screening Tests Market Analysis and Size
Colorectal cancer (CRC) is the third most widespread form of cancer. CRC results for around a tenth of all cancer incidences globally. Regular screening enables early detection, which could result in a 60% reduction in CRC fatalities. Also, with regular annual screening, using in-vitro colorectal cancer screening tests is anticipated to increase the average 5-year survival rate from 46% to 73%. This increasing incidence of colorectal cancer is anticipated to surge the market growth for in-vitro colorectal cancer screening tests.
Data Bridge Market Research analyses the in-vitro colorectal cancer screening tests market growth rate in the 2023-2030. The expected CAGR of the in-vitro colorectal cancer screening tests market is around 7.10% in the mentioned forecast period. The market was valued at USD 943.76 million in 2022 and would grow to USD 1,633.7 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
In-Vitro Colorectal Cancer Screening Tests Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Fecal Occult Blood Test, Biomarker Tests, and CRC DNA Screening Tests), End-User (Hospitals, Clinics, Diagnostics laboratories, and Ambulatory Surgical Centers), Imaging Type (Colonoscopy, Proctoscopy, CT Scan, Ultrasound, MRI, PET Scan), Application (MarCarePlex, Cologic, Colox, miRDIGN, PanCDx, and MeSorce CRC) |
|
Countries Covered |
U.S., Canada, and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Abbott (U.S.), Clinical Genomics Technologies Pty, Ltd (U.S.), Epigenomics AG (Germany), Hemosure, Inc (U.S.), Exact Sciences Corporation (U.S.), NOVIGENIX SA (Switzerland), Sekisui Diagnostics (U.S.), Medline Industries, Inc (U.S.), Beckman Coulter, Inc (U.S.), Eiken Chemical Co, Ltd (Japan), Sysmex Corporation (Japan), Siemens Healthcare Private Limited (Japan), Quest Diagnostics Incorporated (U.S.), Oncocyte Corporation (U.S.), Merck KGaA (Germany), Immunostics Inc (U.S.) |
|
Market Opportunities |
|
Market Definition
Colorectal cancer screening tests in-vitro are widely used to detect the presence of the disease. Colorectal cancer grows in the rectum or colon. It is also called rectal or colon cancer, depending on where it originates. Most colorectal cancers begin as a growth on the inner layer of the rectum or colon. Also, a colonoscopy is one of the most sensitive tests for colon cancer screening.
In-Vitro Colorectal Cancer Screening Tests Market Dynamics
Drivers
- Increased Developments of Colorectal Cancer Screening
Technological advancements in colorectal cancer screening are anticipated to boost market growth during the forecast period. Lately, the FDA approved the fecal DNA test and blood test to detect colorectal cancer to meet the market requirements for minimally invasive diagnostic tests. Furthermore, developments in industrial genetics have used DNA sequencing with high speeds and at affordable costs, enhancing the adoption rate for in-vitro colorectal cancer screening tests, thus increasing market growth. Thus, this is expected to boost market growth.
- Increasing Incidence of Colorectal Cancer
Colorectal cancer (CRC) is the third most common cancer globally, with 1.36 million people affected globally, resulting in approximately 10% of cancers. About 142,820 new cases with 50,830 deaths were accounted for in the U.S. in 2013 because of colorectal cancer, and around 447,000 new cases of CRC and 215,000 deaths in European countries were witnessed in 2012. The new cases of colorectal cancer in China were expected to be around 400000 in 2012. Thus, this increasing number of cancer cases leads to higher screening tests which will help in patient recovery. Thus, this boosts the market growth
Opportunities
- Increasing Number of Genetic Tests
Various genetic tests have been developed for colon cancer, which include the hereditary non-polyposis colon cancer (HNPCC) test and familial adenomatous polyposis (FAP) test. Genetic testing for colon cancer creates much better opportunities for early diagnosis. For instance, the most common genetic changes associated with colon cancer are familial adenomatous polyposis (FAP) and hereditary non-polyposis colorectal cancer (HNPCC). These genetic changes could be identified at a very early age with the help of genetic testing. Thus, this factor boosts market growth.
- Increasing Demand for Colonoscopy
Colonoscopy has been the preferred mode of colorectal cancer screening and prevention for many years. As per the U.S. Preventive Services Task Force, in 2021, the recommended age at which adults are eligible for screening for colorectal cancer is 45-75. The decision to be screened after the age of 75 years should be made on an individual basis. Therefore, many people can be screened for the disease because of the age criteria. Therefore, this factor helps in the growth of the market.
Restraints/Challenges
- High Cost of Screening Tests
Numerous hospitals in underdeveloped and developing nations cannot invest in colorectal cancer screening because of high costs and budget constraints. Though, because of the high demand for diagnostic imaging procedures in these countries, the hospitals that can't afford to buy new and advanced machines for screening opt for other alternatives. Thus, this factor restraints the market growth.
This in-vitro colorectal cancer screening tests market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the In-vitro colorectal cancer screening tests market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In 2021, the U.S. Food and Drug Administration (U.S. FDA) announced the approval of GI Genius, a device that uses artificial intelligence based on machine learning. It is revealed to help clinicians identify lesions such as polyps or suspected tumors in the colon during a colonoscopy.
Global In-Vitro Colorectal Cancer Screening Tests Market Scope
The in-vitro colorectal cancer screening tests market is segmented on the basis of product, imaging type, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Fecal Occult Blood Test
- Guaiac FOB Stool Test
- Immuno-FOB Agglutination Test
- Lateral Flow Immuno-FOB Test
- Immuno-FOB ELISA Test
- Biomarker Tests
- Tumor M2-PK Stool Test
- Transferrin Assasys
- CRC DNA Screening Tests
- Methylated Gene Test
- Panel DNA Test
Imaging Type
- Colonoscopy
- Proctoscopy
- CT Scan
- Ultrasound
- MRI
- PET Scan
End-User
- Hospitals
- Clinics
- Diagnostics laboratories
- Ambulatory Surgical Centers
Application
- MarCarePlex
- Cologic
- Colox
- miRDIGN
- PanCDx
- MeSorce CRC
In-Vitro Colorectal Cancer Screening Tests Market Regional Analysis/Insights
The in-vitro colorectal cancer screening tests market is analyzed and market size insights and trends are provided by product, imaging type, application and end-user as referenced above.
The major countries covered in the in-vitro colorectal cancer screening tests market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the market due to the increasing incidence rate and improved healthcare infrastructure. Factors such as a larger elderly population also led to market growth.
Asia Pacific is projected to witness huge growth in the market to the growing predominance of cancer in this region. Also, cancer is an increasing concern in this region because of the elderly population's expanding base and lifestyle modifications in Asian countries, leading to market growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global In-Vitro Colorectal Cancer Screening Tests Market Share Analysis
The in-vitro colorectal cancer screening tests market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related in-vitro colorectal cancer screening tests market.
Key players operating in the in-vitro colorectal cancer screening tests market include:
- Abbott (U.S.)
- Clinical Genomics Technologies Pty, Ltd (U.S.)
- Epigenomics AG (Germany)
- Hemosure, Inc (U.S.)
- Exact Sciences Corporation (U.S.)
- NOVIGENIX SA (Switzerland)
- Sekisui Diagnostics (U.S.)
- Medline Industries, Inc (U.S.)
- Beckman Coulter, Inc (U.S.)
- Eiken Chemical Co, Ltd (Japan)
- Sysmex Corporation (Japan)
- Siemens Healthcare Private Limited (Japan)
- Quest Diagnostics Incorporated (U.S.)
- Oncocyte Corporation (U.S.)
- Merck KGaA (Germany)
- Immunostics Inc (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。














