Global Hemophilia A Treatment Market
市场规模(十亿美元)
CAGR :
% 
 USD
11.10 Billion
USD
17.77 Billion
2021
2029
USD
11.10 Billion
USD
17.77 Billion
2021
2029
| 2022 –2029 | |
| USD 11.10 Billion | |
| USD 17.77 Billion | |
|
|
|
|
全球血友病 A 治疗市场,按类型(重度、中度、轻度)、产品(重组因子浓缩物、血浆衍生因子浓缩物、延长半衰期产品)、患者(儿科、成人)、诊断(基因检测、纤维蛋白原检测、因子 VIII 和因子 IX 检测、凝血酶原时间 (PT)、全血细胞计数 (CBC))、治疗(预防、按需)、疗法(因子替代疗法、非因子替代疗法)、药物类别(加压素、凝血因子)、给药途径(肠外、鼻喷雾剂、其他)、剂型(注射剂、溶液剂、其他)、最终用户(医院、专科诊所、家庭护理、其他)、分销渠道(医院药房、零售药房、网上药房、其他)– 行业趋势和预测到 2029 年
市场分析和规模
根据美国疾病控制与预防中心 (CDC) 的数据,每 5,617 名活产男婴中就有一名患有血友病。在美国,有 30,000 至 33,000 名男性患有血友病。超过一半的确诊患者患有严重的 A 型血友病。A 型血友病的患病人数是 B 型血友病的四倍。血友病是一种影响所有种族和民族的血液疾病。A 型血友病通常称为经典血友病,是一种因血液中缺乏第八因子而导致的遗传性出血性疾病。
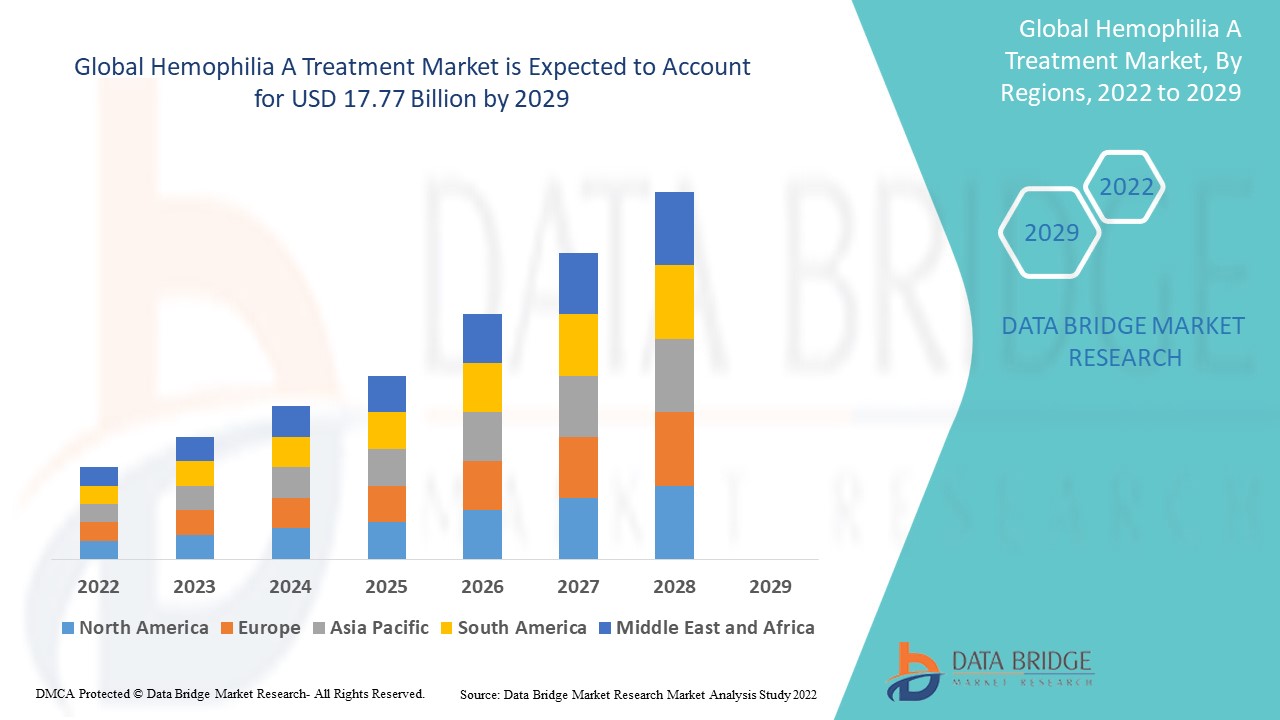
Data Bridge Market Research 分析称,2021 年血友病 A 治疗市场价值为 111 亿美元,预计到 2029 年将达到 177.7 亿美元,在 2022 年至 2029 年的预测期内复合年增长率为 6.06%。Data Bridge Market Research 团队策划的市场报告包括深入的专家分析、患者流行病学、管道分析、定价分析和监管框架。
报告范围和市场细分
|
报告指标 |
细节 |
|
预测期 |
2022 至 2029 年 |
|
基准年 |
2021 |
|
历史岁月 |
2020(可定制为2014-2019) |
|
定量单位 |
收入(单位:十亿美元)、销量(单位:台)、定价(美元) |
|
涵盖的领域 |
类型(重度、中度、轻度)、产品(重组凝血因子浓缩物、血浆衍生凝血因子浓缩物、延长半衰期产品)、患者(儿童、成人)、诊断(基因检测、纤维蛋白原检测、凝血因子 VIII 和凝血因子 IX 检测、凝血酶原时间 (PT)、全血细胞计数 (CBC))、治疗(预防、按需)、疗法(凝血因子替代疗法、非凝血因子替代疗法)、药物类别(加压素、凝血因子)、给药途径(肠胃外、鼻腔喷雾剂、其他)、剂型(注射剂、溶液剂、其他)、最终用户(医院、专科诊所、家庭护理、其他)、分销渠道(医院药房、零售药房、网上药房、其他) |
|
覆盖国家 |
北美洲的美国、加拿大和墨西哥、德国、法国、英国、荷兰、瑞士、比利时、俄罗斯、意大利、西班牙、土耳其、欧洲其他地区、中国、日本、印度、韩国、新加坡、马来西亚、澳大利亚、泰国、印度尼西亚、菲律宾、亚太地区 (APAC) 的其他地区、沙特阿拉伯、阿联酋、南非、埃及、以色列、中东和非洲 (MEA) 的其他地区、巴西、阿根廷和南美洲其他地区 |
|
涵盖的市场参与者 |
辉瑞公司(美国)、葛兰素史克公司(英国)、诺华公司(瑞士)、迈兰公司(美国)、梯瓦制药工业有限公司(以色列)、赛诺菲(法国)、阿斯利康公司(英国)、强生私人有限公司(美国)、默克公司(美国)、罗氏公司(瑞士)、百特(美国)、武田制药有限公司(日本)、Grifols,SA(西班牙)、CSL(美国)、BioMarin(美国)、Spark Therapeutics,Inc.(美国)、中外制药株式会社(日本)、Octapharma AG(瑞士)、Novo Nordisk A/S(丹麦)、Biogen(美国)、基因泰克公司(美国)、拜耳公司(德国)、Ferring BV(瑞士) |
|
市场机会 |
|
市场定义
血友病 A 是一种遗传性出血性疾病,会导致血液凝固异常。血友病 A 患者在受伤、手术或牙科手术后会比平时流更多血。血友病 A 是由一种名为因子 VIII 的蛋白质缺乏引起的。替代疗法是最常见的治疗方法,其中将凝血因子 VIII 轻轻滴入或注射到静脉中。根据美国国家生物技术信息中心 (NCBI) 的数据,Emicizumab 是目前唯一获得许可的非因子疗法,可帮助解决静脉通路困难、频繁出血和其他问题。这种治疗方法还被推荐用于预防服用抑制剂和接受手术的个体的出血。经过最近的改进,这些新药显示出潜在的止血特性,并能够显著减少有或没有抑制剂的血友病患者的出血事件。DDAVP(醋酸去氨加压素)是加压素的合成版本。这种天然抗利尿激素有助于止血,因为它具有临床疗效和安全性,并且有用于静脉和鼻腔给药的浓缩配方。
血友病 A 治疗市场动态
驱动程序
- 全球血友病患病率高
全球血友病患病率的上升将成为导致市场增长率扩大的主要驱动力。重度血友病患者需要频繁接受血友病治疗,以保持血液中有足够的凝血因子,避免出血。包括世界血友病联盟于 2021 年 10 月发表的一项全球研究,全球有 209,614 人被诊断患有血友病,其中 165,379 人为血友病 A 患者。此外,接受优质护理的患者更有可能患上更少的合并症,并降低严重出血的风险。因此,患者数量的不断增加增加了对血友病治疗的需求,这将提高产品采用率。
- 儿科人口数量激增
预计在 2022-2029 年的预测期内,儿科人口数量的增加将提高市场的增长率。儿科血友病的患病率正在稳步上升。根据世界血友病联盟 (WFH) 的数据,预计每 10,000 名新生儿中就有 1 名患有血友病。患有血友病的儿童血液中缺乏足够的凝血因子,因为他们无法止血,因此需要使用特定的血友病药物来防止过度出血。许多政府和非政府组织正在发起关于儿科血友病的症状、诊断和治疗的公众意识运动。此外,知名行业参与者正致力于开发创新解决方案,以尽量减少儿科血友病的严重程度,从而加速市场增长。
此外,公共和私人组织不断增加的宣传活动将扩大甲型血友病治疗市场。此外,人们生活方式的改变和政府日益增多的优惠举措将导致甲型血友病治疗市场扩大。影响甲型血友病治疗市场增长率的另一个重要因素是医疗保健支出的增加,这有助于改善其基础设施。
机会
- 增加研发活动数量
此外,市场的增长得益于研发活动的增加。这将为血友病 A 治疗市场的增长提供有利的机会。BioMarin Pharmaceutical Inc. 于 2021 年表示,它已在美国完成了一项针对重度血友病 A 成人的大型 3 期基因治疗试验。在预测期内,此类持续的研究和创新举措预计将刺激市场需求。因此,上述变量预计将促进该国的产品吸收。
- 新产品发布
在预测期内,血友病 A 治疗市场行业参与者推出的新产品预计将促进新的市场机会。例如,欧盟委员会于 2019 年 6 月批准 Novo Nordisk 在欧洲销售“Esperoct”。Turoctocog alfa pegol,N8-GP,以商品名 Esperoct 销售,用于治疗青少年和成人的血友病 A(先天性第八因子缺乏症)。此外,Roche Products India Pvt. Ltd 于 2019 年 4 月在印度推出了血友病 A 药物 Hemlibra。通过第八因子抑制剂,建议对血友病 A 患者进行预防性治疗,以减少出血发作的频率。
此外,对先进技术开发的投资增加和新兴市场数量的增加将为预测期内血友病 A 治疗市场的增长提供有利机会。
限制/挑战
- 血友病 A 治疗费用高昂
治疗相关的高成本将阻碍 2022-2029 年预测期内血友病 A 市场的增长率。根据美国国家血友病基金会的数据,血友病治疗的平均费用每年超过 30 万美元,这对运营利润率有重大影响,尤其是在当今按人头报销的环境下。由于凝血因子治疗相关的高成本,收入水平相对较低的新兴国家的市场扩张可能会受到阻碍。
另一方面,发展中经济体缺乏医疗基础设施和缺乏熟练的专业人员将对甲型血友病治疗市场构成挑战。此外,严格的政府政策和对治疗缺乏认识将限制并进一步阻碍 2022-2029 年预测期内市场的增长速度。
本血友病 A 治疗市场报告详细介绍了最新发展、贸易法规、进出口分析、生产分析、价值链优化、市场份额、国内和本地市场参与者的影响,分析了新兴收入来源、市场法规变化、战略市场增长分析、市场规模、类别市场增长、应用领域和主导地位、产品批准、产品发布、地域扩展、市场技术创新等方面的机会。如需了解有关血友病 A 治疗市场的更多信息,请联系 Data Bridge Market Research 获取分析师简报,我们的团队将帮助您做出明智的市场决策,实现市场增长。
患者流行病学分析
继血管性血友病之后,血友病 A 是最常见的 X 连锁隐性遗传病,也是最常见的遗传性凝血因子缺乏症。男性更容易患血友病 A,但女性也可能受到影响。每 5,000 名新生男婴中约有 1 人患有血友病 A。血友病 A 影响约 60% 的人。血友病对所有种族和民族的影响相同。
血友病 A 治疗市场还为您提供详细的市场分析,包括患者分析、预后和治疗。患病率、发病率、死亡率、依从率是报告中提供的一些数据变量。分析流行病学对市场增长的直接或间接影响,以创建更稳健、更全面的多元统计模型,用于预测增长期的市场。
COVID-19 对血友病 A 治疗市场的影响
由于全球供应链和物流中断,COVID-19 疫情的爆发对血友病治疗市场产生了轻微的负面影响。冠状病毒疫情对该行业的影响在 2020 年初达到顶峰,当时患有此类疾病的人更容易感染传染病,因此感染冠状病毒的风险更高。另一方面,一些组织、医院和血液学家正在努力应对 COVID-19 公共卫生问题。例如,为了应对冠状病毒疫情,美国血液学会建立了一个论坛,以交流有用、准确和最新的信息,以帮助血液学家。因此,预计这种方法将有助于在 COVID-19 疫情后恢复治疗量。
近期发展
- 2020 年 2 月,诺和诺德宣布推出长效重组凝血因子 VIII 产品 ESPEROCT。该产品用于预防和治疗 A 型血友病患者的出血。新疗法通常用于常规预防,进一步减少出血发作、按需治疗、控制出血发作以及围手术期出血管理。
全球血友病 A 治疗市场范围
血友病 A 治疗市场根据类型、产品、人口统计、治疗、诊断、疗法、药物类别、剂型、给药途径、最终用户和分销渠道进行细分。这些细分市场之间的增长将帮助您分析行业中增长微弱的细分市场,并为用户提供有价值的市场概览和市场洞察,帮助他们做出战略决策,确定核心市场应用。
类型
- 严重
- 缓和
- 温和的
产品
- 重组因子浓缩物
- 第八因子
- 第九因子
- 血浆因子浓缩物
- 第八因子
- 第九因子
- 延长半衰期产品
- 第八因子
- 第九因子
人口统计
- 儿科
- 0 至 4
- 5 至 13
- 14 至 18
- 成人
- 19至44
- 45 岁以上
诊断
- 基因检测
- 纤维蛋白原检测
- 因子 VIII 和因子 IX 测试
- 凝血酶原时间 (PT)
- 全血细胞计数 (CBC)
治疗
- 预防
- 一经请求
治疗
- 因子替代疗法
- 非因子替代疗法
药物类别
- 加压素
- 凝血因子
剂型
- 注射
- 解决方案
- 其他的
给药途径
- 肠外
- 鼻腔喷雾剂
- 其他的
最终用户
- 医院
- 专科诊所
- 家庭护理
- 其他的
分销渠道
- 医院药房
- 零售药店
- 网上药店
- 其他的
血友病 A 治疗市场区域分析/见解
对血友病 A 治疗市场进行了分析,并按国家、类型、产品、人口统计、治疗、诊断、疗法、药物类别、剂型、给药途径、最终用户和分销渠道提供了市场规模洞察和趋势。
血友病 A 治疗市场报告涵盖的国家包括北美洲的美国、加拿大和墨西哥、欧洲的德国、法国、英国、荷兰、瑞士、比利时、俄罗斯、意大利、西班牙、土耳其、欧洲其他地区、亚太地区 (APAC) 的中国、日本、印度、韩国、新加坡、马来西亚、澳大利亚、泰国、印度尼西亚、菲律宾、亚太地区 (APAC) 的其他地区、中东和非洲 (MEA) 的其他地区、南美洲的巴西、阿根廷和南美洲其他地区
北美在市场份额和市场收入方面占据血友病 A 治疗市场的主导地位,并将在预测期内继续保持主导地位。这是由于主要关键参与者的存在,而医疗保健支出的增加将进一步推动该地区市场的增长率。此外,不断增加的研发活动将进一步推动该地区市场的增长率。
由于该地区对血友病 A 新型疗法的批准不断增加,预计亚太地区将在 2022-2029 年预测期内实现增长。此外,医疗保健基础设施的发展和政府举措的不断增加将进一步推动该地区市场的增长率。
报告的国家部分还提供了影响单个市场因素和国内市场监管变化,这些因素和变化会影响市场的当前和未来趋势。下游和上游价值链分析、技术趋势和波特五力分析、案例研究等数据点是用于预测单个国家市场情景的一些指标。此外,在提供国家数据的预测分析时,还考虑了全球品牌的存在和可用性以及它们因来自本地和国内品牌的大量或稀缺竞争而面临的挑战、国内关税和贸易路线的影响。
竞争格局和血友病 A 治疗市场份额分析
The hemophilia A treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to hemophilia A treatment market.
Some of the major players operating in the hemophilia A treatment market are:
- Pfizer Inc. (US)
- GlaxoSmithKline plc (UK)
- Novartis AG (Switzerland)
- Mylan N.V. (US)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- AstraZeneca (UK)
- Johnson & Johnson Private Limited (US)
- Merck & Co., Inc. (US)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Baxter (US)
- Takeda Pharmaceutical Company Limited (Japan)
- Grifols, S.A. (Spain)
- CSL (US)
- BioMarin (US)
- Spark Therapeutics, Inc. (US)
- Chugai Pharmaceutical Co. Ltd. (Japan)
- Octapharma AG (Switzerland)
- Novo Nordisk A/S (Denmark)
- Biogen (US)
- Genentech Inc. (US)
- Bayer AG (Germany)
- Ferring B.V. (Switzerland)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。