Global Generic Drug Market
市场规模(十亿美元)
CAGR :
% 
 USD
622.02 Million
USD
1,323.68 Million
2022
2030
USD
622.02 Million
USD
1,323.68 Million
2022
2030
| 2023 –2030 | |
| USD 622.02 Million | |
| USD 1,323.68 Million | |
|
|
|
|
全球仿製藥市場,按類型(簡單仿製藥、超級仿製藥)、品牌(純仿製藥、品牌仿製藥)、適應症(中樞神經系統 (CNS)、心血管、皮膚病學、腫瘤學、呼吸系統等)、給藥途徑(口服、外用、腸外給藥、其他)、最終用戶(醫院、家庭護理、專科診所、其他零售通路(20 年藥店和零售行業。
仿製藥市場分析及規模
世界衛生組織估計,每年有200萬至300萬例非黑色素瘤皮膚癌和13.2萬例黑色素瘤皮膚癌。此外,牛皮癬在全球的盛行率從0.09%到11.43%不等,這是一種嚴重疾病,影響全球至少1億人。事實上,外用藥物是大多數皮膚病的主要治療方法,預計未來幾年先進外用產品市場將會成長。
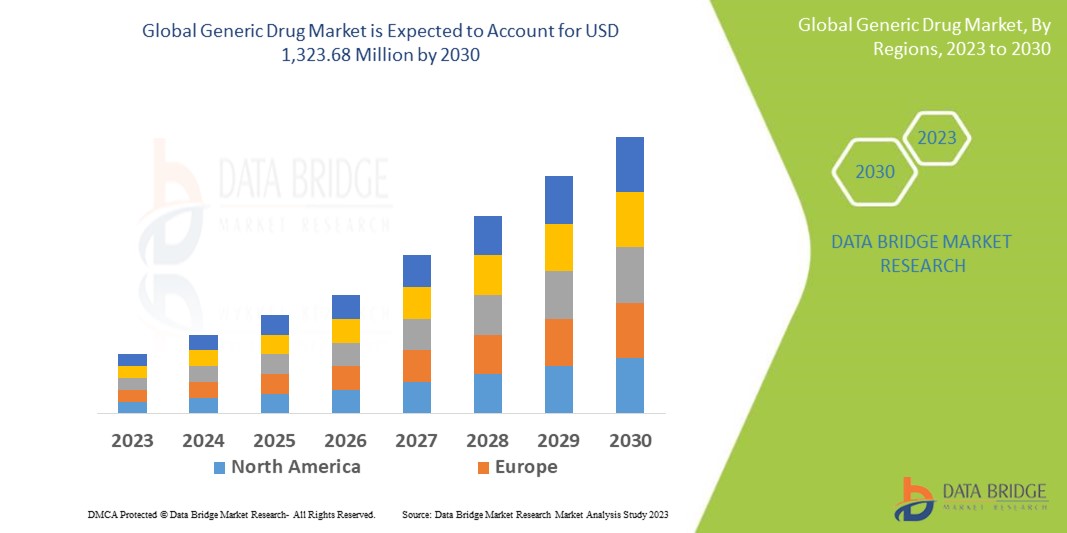
Data Bridge Market Research 分析稱,仿製藥市場規模在 2022 年為 6.2202 億美元,預計到 2030 年將達到 13.2368 億美元,在 2023 年至 2030 年的預測期內複合年增長率為 9.9%。除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察外,Data Bridge Market Research 策劃的市場報告還包括深度專家分析、患者流行病學、管道分析、定價分析和監管框架。
仿製藥市場範圍和細分
|
報告指標 |
細節 |
|
預測期 |
2023年至2030年 |
|
基準年 |
2022 |
|
歷史歲月 |
2021(可自訂為 2015 - 2020 年) |
|
定量單位 |
收入(百萬美元)、銷售(單位)、定價(美元) |
|
涵蓋的領域 |
類型(簡單仿製藥、超級仿製藥)、品牌(純仿製藥、品牌仿製藥)、適應症(中樞神經系統(CNS)、心血管、皮膚病學、腫瘤學、呼吸系統等)、給藥途徑(口服、外用、腸外給藥、其他)、最終用戶(醫院、家庭護理、專科診所、其他)、經銷商(醫院藥房) |
|
覆蓋國家 |
北美洲的美國、加拿大和墨西哥、德國、法國、英國、荷蘭、瑞士、比利時、俄羅斯、義大利、西班牙、土耳其、歐洲其他地區、中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓、亞太地區 (APAC) 的其他地區、沙烏地阿拉伯、阿聯酋、南非、埃及、以色列、中東和非洲 (MEA) 的其他地區、其他地區的歐洲地區 |
|
涵蓋的市場參與者 |
Teva Pharmaceuticals Industries Ltd.(以色列)、Mylan NV(美國)、Novartis AG(瑞士)、Pfizer Inc.(美國)、Sun Pharmaceutical Industries Ltd.(印度)、Fresenius SE & Co. KGaA.(德國)、Lupin(印度)、Endo International plc。 PLC.(英國)、STADA Arzneimittel AG(德國)、Eli Lilly and Company(美國)和 Aspen Holdings(南非) |
|
市場機會 |
|
市場定義
仿製藥與品牌藥在多個方面存在差異,例如藥物研發過程中採用的製造流程、輔料和包裝。儘管如此,它們在強度、劑量、品質、安全性、性能和療效方面與品牌藥生物等效。當已上市藥品的專利到期後,仿製藥即可上市。仿製藥不屬於特定製造商,通常受世界各國政府的監管。
仿製藥市場動態
驅動程式
- 人工智慧 (AI) 技術需求的成長將推動行動市場的成長
機器人流程自動化 (RPA)使用人工智慧(AI) 技術來自動化常規的、基於規則的活動。市場的主要營運參與者可以將更多的時間、精力和資源投入到這種自動化的更高價值任務中。仿製藥市場未來幾年將加速發展的關鍵發展之一是使用 RPA 來確保符合法規和標準。製藥公司經常使用 RPA 和其他業務流程自動化工具來執行大量的研發和生產任務。這些都是推動市場成長的特定因素。
- 印度蓬勃發展的製藥業推動印度仿製藥市場
印度是全球最大的醫藥市場之一,擁有多家頂級製藥公司。根據印度政府投資促進與便利化機構Invest India的數據,印度製藥業在產量方面排名全球第三,在價值方面排名全球第十四。印度不僅擁有3,000多家製藥公司和10,500多家生產設施,還擁有美國境外數量最多的符合美國食品藥物管理局(FDA)標準的製藥廠。
機會
- 癌症病例的增加將成為一個機會
根據國際癌症研究機構 (IARC) 2020 年的報告,該報告估計了全球 185 個國家/地區的 36 種癌症的發病率和死亡率,估計 2020 年全球診斷出 1930 萬例新發癌症病例,其中男性超過 1010 萬例,女性超過 930 萬例。此外,全球 RA 網路 2021 年的研究估計,全球有超過 3.5 億人患有關節炎。由於全球老年人口的增長等多種變量,這一數字可能會繼續上升。因此,預計慢性病盛行率的上升將增加對有效治療的需求,從而在整個預測期內推動仿製藥市場的成長。
限制/挑戰
- 嚴格的監管將阻礙成長
FDA 會評估仿製藥的準確性、副作用和其他成分,而嚴格的管控是限制仿製藥擴張的主要因素之一。如果生產商不遵守監管要求,藥品通常會被召回。影響仿製藥品質的關鍵因素包括純度、效力、穩定性和藥物釋放。這些因素應控制在適當的限度、範圍或分佈範圍內,以達到所需的藥品品質。由於政府的嚴格規定,仿製藥需要獲得批准,預計將阻礙市場擴張。
本仿製藥市場報告詳細介紹了近期發展動態、貿易法規、進出口分析、生產分析、價值鏈優化、市場份額、國內和本地市場參與者的影響,並分析了新興收入來源、市場法規變化、戰略市場增長分析、市場規模、品類市場增長、應用領域和市場主導地位、產品審批、產品上市、地域擴張以及市場技術創新等方面的機遇。如需了解更多關於仿製藥市場的信息,請聯繫 Data Bridge 市場研究部門獲取分析師簡報,我們的團隊將協助您做出明智的市場決策,實現市場成長。
COVID-19對仿製藥市場的影響
新冠疫情對仿製藥市場產生了重大影響。新冠疫情爆發後,幾乎所有企業最初都因各種社交隔離規定而遭遇供應鏈中斷。製藥業也未能倖免,這對仿製藥市場造成了不利影響。之後,由於新冠疫情為治療該疾病的藥物研發提供了更多機會,市場對仿製藥的需求增加。美國食品藥物管理局 (FDA) 設立了仿製藥計畫 (Generic Drug Program),透過發送書面訊息、參與開發早期會議以及在整個申請審查過程中明確監管要求等方式,幫助仿製藥生產商開發新產品。
最新動態
- 2020年,專注於開發、生產和銷售高品質品牌藥和仿製藥的美國綜合專科製藥公司ANI Pharmaceuticals, Inc.以5,250萬美元現金收購了Amerigen Pharmaceuticals, Ltd.的商業及在研仿製藥業務。此次交易大大豐富了ANI Pharmaceuticals, Inc.的商業產品組合和後期仿製藥產品線。
全球仿製藥市場範圍
仿製藥市場根據類型、品牌、適應症、給藥途徑、最終用戶和分銷管道進行細分。這些細分市場的成長將幫助您分析行業中成長緩慢的細分市場,並為用戶提供有價值的市場概覽和市場洞察,幫助他們做出策略決策,確定核心市場應用。
類型
- 簡單泛型
- 超級仿製藥
品牌
- 純通用
- 品牌學名藥
適應症
- 中樞神經系統(CNS)
- 心血管
- 皮膚科
- 腫瘤學
- 呼吸系統
- 其他的
給藥途徑
- 口服
- 外用
- 腸外
- 其他的
最終用戶
- 醫院
- 居家護理
- 專科診所
- 其他的
分銷管道
- 醫院藥房
- 網路藥局
- 零售藥局
仿製藥市場區域分析/洞察
對仿製藥市場進行了分析,並根據上述國家、類型、品牌、適應症、給藥途徑、最終用戶和分銷管道提供了市場規模洞察和趨勢。
仿製藥市場報告涵蓋的國家包括北美洲的美國、加拿大和墨西哥、歐洲的德國、法國、英國、荷蘭、瑞士、比利時、俄羅斯、義大利、西班牙、土耳其、歐洲其他地區、中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓、亞太地區(APAC)的其他地區、沙烏地阿拉伯、阿聯酋、南非、埃及、泰國、中東和其他地區(阿根廷地區的其他地區)。
北美在仿製藥市場佔據主導地位,因為該地區已建立仿製藥審批流程框架,並在研發活動方面處於全球領先地位。
預計亞太地區將在2023年至2030年的預測期內實現最高成長率,這得益於醫療基礎設施的不斷擴張和政府舉措的不斷加強。此外,民眾對醫療狀況的認知度不斷提高,以及該地區人口老化加劇。在亞太地區,中國和印度等國的貢獻超過其他國家。
報告的國家部分還提供了各個市場的影響因素以及國內市場監管變化,這些變化會影響市場的當前和未來趨勢。下游和上游價值鏈分析、技術趨勢、波特五力模型分析以及案例研究等數據點是預測各國市場狀況的一些指標。此外,在對國家/地區數據進行預測分析時,還考慮了全球品牌的存在和可用性,以及它們因本土和國內品牌的激烈競爭或稀缺而面臨的挑戰,國內關稅和貿易路線的影響。
競爭格局和仿製藥市場份額分析
仿製藥市場競爭格局按競爭對手提供詳細資訊。詳細資訊包括公司概況、公司財務狀況、收入、市場潛力、研發投入、新市場計劃、全球佈局、生產基地和設施、生產能力、公司優勢和劣勢、產品發布、產品寬度和廣度以及應用主導地位。以上提供的數據僅與公司在仿製藥市場的重點相關。
仿製藥市場的一些主要參與者包括:
- Teva Pharmaceuticals Industries Ltd.(以色列)
- Mylan NV,(美國)
- 諾華公司(瑞士)
- 輝瑞公司(美國)
- 太陽製藥工業有限公司(印度)
- Fresenius SE & Co. KGaA.,(德國)
- 魯冰花(印度)
- Endo International plc.,(愛爾蘭)
- 奧羅賓多製藥(印度)
- 諾華公司(瑞士)
- Hikma Pharmaceuticals PLC.,(英國)
- STADA Arzneimittel AG(德國)
- 禮來公司(美國)
- 阿斯彭控股(南非)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL GENERIC DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL GENERIC DRUGS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL GENERIC DRUGS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUGS ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATEINT TREATMENT SUCCESS RATES
7 INDUSTRY INSIGHTS
7.1 PATENT ANALYSIS
7.2 DRUGS TREATMENT RATE BY MATURED MARKETS
7.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
7.4 PATIENT FLOW DIAGRAM
7.5 KEY PRICING STRATEGIES
7.6 KEY PATIENT ENROLLMENT STRATEGIES
7.7 INTERVIEWS WITH FORMULATION CHEMIST
7.8 OTHER KOL SNAPSHOTS
8 PIPELINE ANALYSIS
8.1 CLINICAL TRIALS AND PHASE ANALYSIS
8.2 DRUGS THERAPY PIPELINE
8.3 PHASE III CANDIDATES
8.4 PHASE II CANDIDATES
8.5 PHASE I CANDIDATES
8.6 OTHERS (PRE-CLINICAL AND RESEARCH)
9 REGULATORY FRAMEWORK
10 GLOBAL GENERIC DRUGS MARKET, BY TYPE
10.1 OVERVIEW
10.2 SIMPLE GENERICS
10.3 SUPER GENERICS
11 GLOBAL GENERIC DRUGS MARKET, BY BRAND
11.1 OVERVIEW
11.2 PURE GENERIC
11.3 BRANDED GENERIC
12 GLOBAL GENERIC DRUGS MARKET, BY INDICATION
(NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUBSEGMENTS OF INDICATION)
12.1 OVERVIEW
12.2 CANCER DRUGS
12.2.1 ANTITUMOR ANTIBIOTICS
12.2.1.1. DAUNORUBICIN
12.2.1.1.1. MARKET VALUE(USD MILLION)
12.2.1.1.2. MARKET VOLUME(UNITS)
12.2.1.1.3. AVERAGE SELLING PRICE(ASP)
12.2.1.2. DOXORUBICIN
12.2.1.3. IDARUBICIN
12.2.1.4. MITOXANTRONE
12.2.1.5. OTHERS
12.2.2 DNA-DAMAGING AGENTS
12.2.2.1. CHLORAMBUCIL
12.2.2.2. CYCLOPHOSPHAMIDE
12.2.2.3. MELPHALAN
12.2.2.4. CARBOPLATIN
12.2.2.5. OTHERS
12.2.3 ANTIMETABOLITES
12.2.3.1. METHOTREXATE
12.2.3.2. FLUDARABINE
12.2.3.3. CYTARABINE
12.2.3.4. OTHERS
12.2.4 DNA-REPAIR ENZYME INHIBITORS
12.2.4.1. ETOPOSIDE
12.2.4.2. TOPOTECAN
12.2.4.3. OTHERS
12.2.5 ANTIMITOTIC DRUGS
12.2.5.1. VINCRISTINE
12.2.5.2. VINBLASTINE
12.2.5.3. OTHERS
12.2.6 OTHERS
12.3 CENTRAL NERVOUS SYSTEM PRODUCT
12.3.1 CHOLINESTERASE INHIBITORS
12.3.1.1. RIVASTIGMINE
12.3.1.2. DONEPEZIL
12.3.1.3. GALANTAMINE
12.3.1.4. TACRINE
12.3.1.5. OTHERS
12.3.2 NMDA RECEPTOR ANTAGONISTS
12.3.2.1. KETAMINE
12.3.2.2. DEXTROMETHORPHAN
12.3.2.3. MEMANTINE
12.3.2.4. AMANTADINE
12.3.3 ANTIEPILEPTIC
12.3.3.1. FIRST GENERATION
12.3.3.1.1. PHENOBARBITAL
12.3.3.1.2. PHENYTOIN
12.3.3.1.3. PRIMIDONE
12.3.3.1.4. ETHOSUXIMIDE
12.3.3.1.5. CARBAMAZEPINE
12.3.3.1.6. CLOBAZAM
12.3.3.2. SECOND GENERATION
12.3.3.2.1. VIGABATRIN
12.3.3.2.2. LAMOTRIGINE
12.3.3.2.3. GABAPENTIN
12.3.3.2.4. TOPIRAMATE
12.3.3.2.5. PREGABALIN
12.3.3.2.6. OTHERS
12.3.3.3. THIRD GENERATION
12.3.3.3.1. LACOSAMIDE
12.3.3.3.2. RUFINAMIDE
12.3.3.3.3. PERAMPANEL
12.3.3.3.4. OTHERS
12.3.4 ANTIPSYCHOTIC
12.3.4.1. TYPICAL ANTIPSYCHOTIC
12.3.4.1.1. HALOPERIDOL
12.3.4.1.2. LOXAPINE
12.3.4.1.3. THIORIDAZINE
12.3.4.1.4. MOLINDONE
12.3.4.1.5. THIOTHIXENE
12.3.4.1.6. FLUPHENAZINE
12.3.4.1.7. MESORIDAZINE
12.3.4.1.8. TRIFLUOPERAZINE
12.3.4.1.9. PERPHENAZINE
12.3.4.1.10. CHLORPROMAZINE
12.3.4.2. ATYPICAL ANTIPSYCHOTIC
12.3.4.2.1. ARIPIPRAZOLE
12.3.4.2.2. CLOZAPINE
12.3.4.2.3. ZIPRASIDONE
12.3.4.2.4. RISPERIDONE
12.3.4.2.5. QUETIAPINE
12.3.4.2.6. OLANZAPINE
12.3.5 OTHERS
12.4 DERMATOLOGICAL PRODUCTS
12.4.1 CORTICOSTEROIDS, BY TYPE
12.4.1.1. TOPICAL
12.4.1.1.1. MOMETASONE
12.4.1.1.2. BETAMETHASONE
12.4.1.1.3. HYDROCORTISONE
12.4.1.1.4. FLUTICASONE PROPIONATE
12.4.1.1.5. ALCLOMETASONE
12.4.1.1.6. TRIAMCINOLONE
12.4.1.1.7. FLUOCINOLONE ACETONIDE
12.4.1.1.8. CLOBETASOL PROPIONATE
12.4.1.1.9. OTHERS
12.4.1.2. ORAL
12.4.1.2.1. PREDNISOLONE
12.4.1.2.2. PREDNISONE
12.4.1.2.3. CORTISONE
12.4.1.2.4. METHYLPREDNISOLONE
12.4.1.2.5. OTHERS
12.4.2 RETINOIDS
12.4.2.1. ACITRETIN
12.4.2.2. ADAPALENE
12.4.2.3. ISOTRETINOIN
12.4.2.4. OTHERS
12.4.3 ANTIHISTAMINES AGENTS
12.4.3.1. CYPROHEPTADINE
12.4.3.2. DIPHENHYDRAMINE
12.4.3.3. HYDROXYZINE
12.4.3.4. OTHERS
12.4.4 CALCINEURIN INHIBITORS
12.4.4.1. TACROLIMUS
12.4.4.2. PIMECROLIMUS
12.4.5 ANTIINFECTIVES
12.4.5.1. ANTIBIOTICS
12.4.5.1.1. DOXYCYCLINE
12.4.5.1.2. RETAPAMULIN
12.4.5.1.3. DELAFLOXACIN
12.4.5.1.4. MINOCYCLINE
12.4.5.1.5. MUPIROCIN
12.4.5.1.6. OTHERS
12.4.5.2. ANTIFUNGAL
12.4.5.2.1. SYNTHETIC
12.4.5.2.1.1 CLOTRIMAZOLE
12.4.5.2.1.2 MICONAZOLE
12.4.5.2.1.3 OTHERS
12.4.5.2.2. IMIDAZOLES DRUGS
12.4.5.2.2.1 KETOCONAZOLE
12.4.5.2.2.2 CLONAZEPAM
12.4.5.2.3. OTHERS
12.4.5.2.4. OTHERS
12.4.5.3. ANTIVIRAL
12.4.5.3.1. ACYCLOVIR
12.4.5.3.2. FAMCICLOVIR
12.4.5.3.3. VALACYCLOVIR
12.4.5.3.4. OTHERS
12.4.5.4. OTHERS
12.4.6 HAIR GROWTH
12.4.6.1. MINOXIDIL
12.4.6.2. FINASTERIDE
12.4.6.3. SPIRONOLACTONE
12.4.6.4. DUTASTERIDE
12.4.6.5. OTHERS
12.4.7 OTHERS
12.5 GASTROINTESTINAL PRODUCTS
12.5.1 LAXATIVES
12.5.1.1. OSMOTIC LAXATIVES
12.5.1.1.1. GOLYTELY
12.5.1.1.2. COLYTE
12.5.1.1.3. MACROGOL 400
12.5.1.2. STIMULANT LAXATIVES
12.5.1.2.1. BISACODYL
12.5.1.2.2. CASTOR OIL
12.5.1.2.3. PHENOLPHTHALEIN
12.5.1.2.4. SENNA
12.5.1.3. BULK LAXATIVES
12.5.1.3.1. PSYLLIUM
12.5.1.3.2. METHYL CELLULOSE
12.5.1.3.3. POLYCARBOPHIL
12.5.1.3.4. OTHERS
12.5.1.4. LUBRICANT & EMOLLIENT LAXATIVES
12.5.1.4.1. MINERAL OIL
12.5.1.4.2. GLYCERIN SUPPOSITORIES
12.5.1.4.3. OTHERS
12.5.2 ANTACIDS
12.5.2.1. SODIUM ANTACIDS
12.5.2.2. CALCIUM ANTACIDS
12.5.2.3. MAGNESIUM ANTACIDS
12.5.2.4. ALUMINIUM ANTACIDS
12.5.2.5. OTHERS
12.5.3 ANTIDIARRHEALS
12.5.3.1. DIPHENOXYLATE
12.5.3.2. LOPERAMIDE
12.5.3.3. CODEINE
12.5.3.4. OTHERS
12.5.4 H2 BLOCKERS
12.5.4.1. FAMOTIDINE
12.5.4.2. RANITIDINE
12.5.4.3. OTHERS
12.5.5 PROTON PUMP INHIBITORS
12.5.5.1. OMEPRAZOLE
12.5.5.2. LANSOPRAZOLE
12.5.5.3. OTHERS
12.5.6 BILE ACID SEQUESTRANTS
12.5.6.1. CHOLESTYRAMINE
12.5.6.2. COLESTIPOL
12.5.7 OTHERS
12.6 RESPIRATORY PRODUCT
12.6.1 BRONCHODILATORS
12.6.1.1. ALBUTEROL
12.6.1.2. LEVALBUTEROL
12.6.1.3. SALMETEROL
12.6.1.4. FORMOTEROL
12.6.1.5. OTHERS
12.6.2 CORTICOSTEROIDS
12.6.2.1. RACEMIC EPINEPHRINE
12.6.2.2. FLUTICASONE
12.6.2.3. BUDESONIDE
12.6.2.4. OTHERS
12.6.3 MAST CELL STABILIZERS
12.6.3.1. MOMETASONE FUROATE
12.6.3.2. NEDOCROMIL
12.6.3.3. OTHERS
12.6.4 LEUKOTRIENE RECEPTOR ANTAGONISTS
12.6.4.1. CROMOLYN SODIUM
12.6.4.2. OMALIZUMAB
12.6.4.3. OTHERS
12.6.5 ANTIHISTAMINES
12.6.5.1. ZAFIRLUKAST
12.6.5.2. MONTELUKAST
12.6.5.3. ZILEUTON
12.6.5.4. OTHERS
12.6.6 RESPIRATORY STIMULANTS
12.6.6.1. LORATIDINE
12.6.6.2. FEXOFENADINE
12.6.6.3. CETIRIZINE
12.6.6.4. EPINEPHRINE
12.6.6.5. OTHERS
12.6.7 PULMONARY SURFACTANTS
12.6.7.1. DOXAPRAM
12.6.7.2. THEOPHYLLINE
12.6.7.3. PROGESTERONE
12.6.7.4. CAFFEINE
12.6.7.5. OTHERS
12.6.8 OXYGEN ANTIMICROBIALS
12.6.8.1. COLFOSCERIL PALMITATE
12.6.8.2. BERACTANT
12.6.8.3. CALFACTANT
12.6.8.4. PORACTANT ALFA
12.6.8.5. OTHERS
12.6.9 OTHERS
12.7 OPHTHALMIC PRODUCTS
12.7.1 ARTIFICIAL TEARS
12.7.1.1. DEMULCENT
12.7.1.1.1. POLYETHYLENE GLYCOL (PEG)
12.7.1.1.2. PROPYLENE GLYCOL
12.7.1.1.3. GLYCERIN
12.7.1.1.4. POLYVINYL ALCOHOL (PVA)
12.7.1.1.5. OTHERS
12.7.1.2. EMOLLIENTS
12.7.1.2.1. PARAFFIN
12.7.1.2.2. ANHYDROUS LANOLIN
12.7.1.2.3. WHITE WAX
12.7.1.2.4. OTHERS
12.7.1.3. OTHERS
12.7.2 NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
12.7.2.1. BROMFENAC
12.7.2.2. KETOROLAC
12.7.2.3. DICLOFENAC
12.7.2.4. OTHERS
12.7.3 CORTICOSTEROID
12.7.3.1. PREDNISOLONE
12.7.3.2. LOTEPREDNOL
12.7.3.3. FLUOROMETHOLONE
12.7.3.4. OTHERS
12.7.4 BETA BLOCKERS
12.7.4.1. LEVOBUNOLOL
12.7.4.2. TIMOLOL
12.7.4.3. BETAXOLOL
12.7.4.4. OTHERS
12.7.5 PROSTAGLANDIN ANALOGS
12.7.5.1. BIMATOPROST
12.7.5.2. TRAVOPROST
12.7.5.3. LATANOPROST
12.7.5.4. OTHERS
12.7.6 CARBONIC ANHYDRASE INHIBITORS
12.7.6.1. BRINZOLAMIDE
12.7.6.2. DORZOLAMIDE
12.7.7 COMBINATION DRUGS
12.7.7.1. BRIMONIDINE/TIMOLOL
12.7.7.2. DORZOLAMIDE/TIMOLOL
12.7.7.3. PHENYLEPHRINE
12.7.7.4. PROPARACAINE
12.7.7.5. TROPICAMIDE
12.7.7.6. OTHERS
12.8 CARDIOVASCULAR DRUGS
12.8.1 ACE INHIBITORS
12.8.1.1. BENAZEPRIL
12.8.1.2. CAPTOPRIL
12.8.1.3. ENALAPRIL MALEATE
12.8.1.4. LISINOPRIL
12.8.1.5. OTHERS
12.8.2 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
12.8.2.1. CANDESARTAN CILEXETIL
12.8.2.2. EPROSARTAN MESYLATE
12.8.2.3. IRBESARTAN
12.8.2.4. LOSARTAN
12.8.2.5. OTHERS
12.8.3 ANTIARRHYTHMICS
12.8.3.1. AMIODARONE
12.8.3.2. DISOPYRAMIDE PHOSPHATE
12.8.3.3. DOFETILIDE
12.8.3.4. FLECAINIDE
12.8.3.5. MEXILETINE HCL
12.8.3.6. PROCAINAMIDE
12.8.3.7. OTHERS
12.8.4 ANTICOAGULANTS
12.8.4.1. NON-VKA ORAL ANTICOAGULANTS (NOACS)
12.8.4.1.1. RIVAROXABAN
12.8.4.1.2. EDOXABAN
12.8.4.1.3. APIXABAN
12.8.4.1.4. OTHERS
12.8.4.2. HEPARIN & LMWH
12.8.4.2.1. DALTEPARIN
12.8.4.2.2. ENOXAPARIN
12.8.4.2.3. TINZAPARIN
12.8.4.2.4. OTHERS
12.8.4.3. VITAMIN K ANTAGONIST
12.8.4.3.1. WARFARIN
12.8.4.3.2. PHENPROCOUMON
12.8.4.3.3. OTHERS
12.8.4.4. THROMBIN INHIBITORS
12.8.4.4.1. BIVALIRUDIN
12.8.4.4.2. ARGATROBAN
12.8.4.4.3. DABIGATRAN
12.8.4.4.4. OTHERS
12.8.4.5. OTHERS
12.8.5 PLATELET INHIBITORS
12.8.5.1. ASPIRIN
12.8.5.2. CILOSTAZOL
12.8.5.3. CLOPIDOGRIL BISULFATE
12.8.5.4. DIPYRAMIDAMOLE
12.8.5.5. OTHERS
12.8.6 ANTIHYPERTENSIVES
12.8.6.1. CLONIDINE HCL
12.8.6.2. DOXAZOSIN MESYLATE
12.8.6.3. HYDRALAZINE HCI
12.8.6.4. METHYLDOPA
12.8.6.5. MINOXIDIL
12.8.6.6. OTHERS
12.8.7 BETA BLOCKERS
12.8.7.1. ACEBUTOLOL HCL
12.8.7.2. ATENOLOL
12.8.7.3. BETAXOLOL
12.8.7.4. BISOPROLOL
12.8.7.5. CARVEDILOL
12.8.7.6. LABETALOL HCL
12.8.7.7. METOPROLOL
12.8.7.8. METOPROLOL
12.8.7.9. NADOLOL
12.8.7.10. OTHERS
12.8.8 CALCIUM CHANNEL BLOCKERS
12.8.8.1. DIHYDROPYRIDINES
12.8.8.1.1. AMLODIPINE BESYLATE
12.8.8.1.2. NIFEDIPINE
12.8.8.1.3. NIMODIPINE
12.8.8.1.4. NISOLDIPINE
12.8.8.1.5. NICARDIPINE HCL
12.8.8.2. NONDIHYDROPYRIDINES
12.8.8.2.1. DILTIAZEM HCL
12.8.8.2.2. VERAPAMIL HCL
12.8.9 DIURETICS
12.8.9.1. THIAZIDE DIURETICS
12.8.9.1.1. CHLORTHALIDONE
12.8.9.1.2. HYDROCHLOROTHIAZIDE
12.8.9.1.3. METOLAZONE
12.8.9.1.4. INDAPAMIDE
12.8.9.2. LOOP DIURETICS
12.8.9.2.1. TORSEMIDE
12.8.9.2.2. FUROSEMIDE
12.8.9.2.3. BUMETANIDE
12.8.9.3. POTASSIUM-SPARING DIURETICS
12.8.9.3.1. AMILORIDE
12.8.9.3.2. TRIAMTERENE
12.8.9.3.3. SPIRONOLACTONE
12.8.9.3.4. EPLERENONE
12.8.9.4. OTHERS
12.8.10 LIPID MEDICATIONS
12.8.10.1. STATINS
12.8.10.1.1. ATORVASTATIN CALCIUM
12.8.10.1.2. FLUVASTATIN SODIUM
12.8.10.1.3. LOVASTATIN
12.8.10.1.4. OTHERS
12.8.10.2. FIBRATES
12.8.10.2.1. FENOFIBRATE
12.8.10.2.2. GEMFIBROZIL
12.8.10.3. BILE ACID SEQUESTRANTS
12.8.10.3.1. COLESEVELAM HCL
12.8.10.3.2. CHOLESTYRAMINE
12.8.10.3.3. COLESTIPOL HCL
12.8.10.4. OTHER LIPID MEDICATIONS
12.8.11 NITRATES
12.8.11.1. ORAL NITROGLYCERIN
12.8.11.2. NITROGLYCERIN OINTMENT
12.8.11.3. NITROGLYCERIN SKIN PATCHES
12.8.11.4. NITROGLYCERIN SUBLINGUAL TABLETS
12.8.11.5. OTHER NITROGLYCERIN TABLETS, CAPSULES, AND SPRAYS
12.8.12 OTHERS
12.9 VITAMIN
12.9.1 VITAMIN B
12.9.2 VITAMIN E
12.9.3 VITAMIN D
12.9.4 VITAMIN C
12.9.5 VITAMIN A
12.9.6 VITAMIN K
12.1 MINERALS
12.10.1 CALCIUM
12.10.2 MAGNESIUM
12.10.3 IRON
12.10.4 POTASSIUM
12.10.5 ZINC
12.10.6 CHROMIUM
12.10.7 SELENIUM
12.10.8 OTHERS
12.11 OTHERS
13 GLOBAL GENERIC DRUGS MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 ORAL
13.2.1 SOLID
13.2.1.1. SOLID, BY TYPE
13.2.1.1.1. TABLETS
13.2.1.1.2. CAPSULES
13.2.1.1.3. POWDER
13.2.1.1.4. PILLS
13.2.1.1.5. OTHERS
13.2.1.2. SOLID , BY DOSE
13.2.1.2.1. LESS THAN 100 MG
13.2.1.2.2. 100- 5OO MG
13.2.1.2.3. 500 MG – 1000 MG
13.2.1.2.4. MORE THAN 1000 MG
13.2.2 SEMI-SOLID
13.2.2.1. SEMI-SOLID, BY TYPE
13.2.2.1.1. GELS
13.2.2.1.2. EMULSIONS
13.2.2.1.3. ELIXIRS
13.2.2.1.4. OTHERS
13.2.2.2. SEMI-SOLID, BY DOSE
13.2.2.2.1. LESS THAN 25 ML
13.2.2.2.2. 25-50 ML
13.2.2.2.3. MORE THAN 50 ML
13.2.3 LIQUID
13.2.3.1. LIQUID, BY TYPE
13.2.3.1.1. SOLUTIONS
13.2.3.1.2. SYRUPS
13.2.3.1.3. OTHERS
13.2.3.2. LIQUID, BY DOSE
13.2.3.2.1. LESS THEN 25 ML
13.2.3.2.2. 25-50 ML
13.2.3.2.3. MORE THAN 50 ML
13.3 TOPICAL
13.3.1 LIQUID
13.3.1.1. LIQUID, BY TYPE
13.3.1.1.1. SOLUTIONS
13.3.1.1.2. SUSPENSIONS
13.3.1.2. LIQUID, BY DOSE
13.3.1.2.1. LESS THEN 25 ML
13.3.1.2.2. 25-50 ML
13.3.1.2.3. MORE THAN 50 ML
13.3.2 SEMI-SOLID
13.3.2.1. SEMI-SOLID, BY TYPE
13.3.2.1.1. CREAM
13.3.2.1.2. OINTMENT
13.3.2.1.3. GELS
13.3.2.1.4. PASTES
13.3.2.1.5. LOTIONS
13.3.2.1.6. OTHERS
13.3.2.2. SEMI-SOLID, BY DOSE
13.3.2.2.1. LESS THAN 25 MG
13.3.2.2.2. 25-50 MG
13.3.2.2.3. MORE THAN 50 MG
13.3.3 SOLID
13.3.3.1. SOLID, BY TYPE
13.3.3.1.1. SUPPOSITORIES
13.3.3.1.2. POWDERS
13.3.3.2. SOLID, BY DOSE
13.3.3.2.1. LESS THAN 1GM
13.3.3.2.2. 1GM
13.3.3.2.3. MORE THAN 1GM
13.3.4 OTHERS
13.4 PARENTERAL
13.4.1 PARENTERAL, BY TYPE
13.4.1.1. CONVENTIONAL DRUGS DELIVERY FORMULATIONS
13.4.1.1.1. SOLUTIONS
13.4.1.1.2. RECONSTITUTED/LYOPHILIZED
13.4.1.1.3. SUSPENSIONS
13.4.1.1.4. EMULSIONS
13.4.1.1.5. OTHERS
13.4.1.2. NOVEL DRUGS DELIVERY FORMULATIONS
13.4.1.2.1. COLLOIDAL DISPERSIONS
13.4.1.2.2. MICROPARTICLES
13.4.1.2.3. LONG ACTING INJECTION FORMULATION
13.4.2 PARENTERAL, BY DOSE
13.4.2.1. 1 MG/ML
13.4.2.2. 1-5 MG/ML
13.4.2.3. MORE THAN 5 MG/ML
13.5 OCCULAR
13.5.1 OCCULAR, BY TYPE
13.5.1.1. LIQUID
13.5.1.1.1. EYE DROPS
13.5.1.1.2. SPRAYS
13.5.1.2. SEMI-SOLID
13.5.1.2.1. GELS
13.5.1.2.2. OINTMENTS
13.5.2 OCCULAR, BY DOSE
13.5.2.1. 2.5ML
13.5.2.2. 5ML
13.5.2.3. 10 ML
13.6 INTRANASAL
13.6.1 INTRANASAL, BY TYPE
13.6.1.1. DROPS
13.6.1.2. SPRAYS
13.6.1.3. POWDERS
13.6.1.4. GELS
13.6.1.5. OTHERS
13.6.2 INTRANASAL, BY DOSE
13.6.2.1. 10 ML
13.6.2.2. 20 ML
13.6.3 OTHERS
13.6.4
14 GLOBAL GENERIC DRUGS MARKET, BY POPULATION TYPE
14.1 OVERVIEW
14.2 PEDIATRIC
14.3 ADULT
14.4 GERIATRIC
15 GLOBAL GENERIC DRUGS MARKET, BY MODE OF PURCHASE
15.1 OVERVIEW
15.2 OVER THE COUNTER
15.3 PRESCRIPTION
16 GLOBAL GENERIC DRUGS MARKET, BY END USER
16.1 OVERVIEW
16.2 HOSPITALS
16.2.1 PRIVATE
16.2.2 PUBLIC
16.3 SPECIALTY CLINICS
16.4 HOME HEALTHCARE
16.5 AMBULATORY SURGICAL CENTERS
16.6 COMMUNITY CENTRE
16.7 OTHERS
17 GLOBAL GENERIC DRUGS MARKET, BY DISTRIBUTION CHANNEL
17.1 OVERVIEW
17.2 DIRECT TENDER
17.3 RETAIL SALES
17.3.1 HOSPITAL PHARMACIES
17.3.2 RETAIL PHARMACIES
17.3.3 OTHER
17.4 ONLINE PHARMACIES
17.5 OTHER
18 GLOBAL GENERIC DRUGS MARKET, COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18.2 COMPANY SHARE ANALYSIS: EUROPE
18.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
18.4 COMPANY SHARE ANALYSIS: SOUTH AMERICA
18.5 MERGERS & ACQUISITIONS
18.6 NEW PRODUCT DEVELOPMENT & APPROVALS
18.7 EXPANSIONS
18.8 REGULATORY CHANGES
18.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
19 GLOBAL GENERIC DRUGS MARKET, BY REGION
19.1 GLOBAL GENERIC DRUGS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
19.2 NORTH AMERICA
19.2.1 U.S.
19.2.2 CANADA
19.2.3 MEXICO
19.3 EUROPE
19.3.1 GERMANY
19.3.2 U.K.
19.3.3 ITALY
19.3.4 FRANCE
19.3.5 SPAIN
19.3.6 RUSSIA
19.3.7 SWITZERLAND
19.3.8 TURKEY
19.3.9 BELGIUM
19.3.10 NETHERLANDS
19.3.11 DENMARK
19.3.12 SWEDEN
19.3.13 POLAND
19.3.14 NORWAY
19.3.15 FINLAND
19.3.16 REST OF EUROPE
19.4 ASIA-PACIFIC
19.4.1 JAPAN
19.4.2 CHINA
19.4.3 SOUTH KOREA
19.4.4 INDIA
19.4.5 SINGAPORE
19.4.6 THAILAND
19.4.7 INDONESIA
19.4.8 MALAYSIA
19.4.9 PHILIPPINES
19.4.10 AUSTRALIA
19.4.11 NEW ZEALAND
19.4.12 VIETNAM
19.4.13 TAIWAN
19.4.14 REST OF ASIA-PACIFIC
19.5 SOUTH AMERICA
19.5.1 BRAZIL
19.5.2 ARGENTINA
19.5.3 REST OF SOUTH AMERICA
19.6 MIDDLE EAST AND AFRICA
19.6.1 SOUTH AFRICA
19.6.2 EGYPT
19.6.3 BAHRAIN
19.6.4 UNITED ARAB EMIRATES
19.6.5 KUWAIT
19.6.6 OMAN
19.6.7 QATAR
19.6.8 SAUDI ARABIA
19.6.9 REST OF MEA
19.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
20 GLOBAL GENERIC DRUGS MARKET, COMPANY PROFILE
20.1 BAYER
20.1.1 COMPANY OVERVIEW
20.1.2 REVENUE ANALYSIS
20.1.3 GEOGRAPHIC PRESENCE
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPEMENTS
20.2 SANOFI
20.2.1 COMPANY OVERVIEW
20.2.2 REVENUE ANALYSIS
20.2.3 GEOGRAPHIC PRESENCE
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPEMENTS
20.3 PFIZER, INC.
20.3.1 COMPANY OVERVIEW
20.3.2 REVENUE ANALYSIS
20.3.3 GEOGRAPHIC PRESENCE
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPEMENTS
20.4 GLAXOSMITHKLINE PLC
20.4.1 COMPANY OVERVIEW
20.4.2 REVENUE ANALYSIS
20.4.3 GEOGRAPHIC PRESENCE
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPEMENTS
20.5 TAKEDA PHARMACEUTICAL COMPANY LTD
20.5.1 COMPANY OVERVIEW
20.5.2 REVENUE ANALYSIS
20.5.3 GEOGRAPHIC PRESENCE
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPEMENTS
20.6 ABBOTT
20.6.1 COMPANY OVERVIEW
20.6.2 REVENUE ANALYSIS
20.6.3 GEOGRAPHIC PRESENCE
20.6.4 PRODUCT PORTFOLIO
20.6.5 RECENT DEVELOPEMENTS
20.7 NOVARTIS AG
20.7.1 COMPANY OVERVIEW
20.7.2 REVENUE ANALYSIS
20.7.3 GEOGRAPHIC PRESENCE
20.7.4 PRODUCT PORTFOLIO
20.7.5 RECENT DEVELOPEMENTS
20.8 SUN PHARMACEUTICAL INDUSTRIES
20.8.1 COMPANY OVERVIEW
20.8.2 REVENUE ANALYSIS
20.8.3 GEOGRAPHIC PRESENCE
20.8.4 PRODUCT PORTFOLIO
20.8.5 RECENT DEVELOPEMENTS
20.9 TEVA PHARMACEUTICAL INDUSTRIES LTD.
20.9.1 COMPANY OVERVIEW
20.9.2 REVENUE ANALYSIS
20.9.3 GEOGRAPHIC PRESENCE
20.9.4 PRODUCT PORTFOLIO
20.9.5 RECENT DEVELOPEMENTS
20.1 DR. REDDY’S LABORATORIES
20.10.1 COMPANY OVERVIEW
20.10.2 REVENUE ANALYSIS
20.10.3 GEOGRAPHIC PRESENCE
20.10.4 PRODUCT PORTFOLIO
20.10.5 RECENT DEVELOPEMENTS
20.11 ENDO PHARMACEUTICALS INC.
20.11.1 COMPANY OVERVIEW
20.11.2 REVENUE ANALYSIS
20.11.3 GEOGRAPHIC PRESENCE
20.11.4 PRODUCT PORTFOLIO
20.11.5 RECENT DEVELOPEMENTS
20.12 AMNEAL PHARMACEUTICALS INC
20.12.1 COMPANY OVERVIEW
20.12.2 REVENUE ANALYSIS
20.12.3 GEOGRAPHIC PRESENCE
20.12.4 PRODUCT PORTFOLIO
20.12.5 RECENT DEVELOPEMENTS
20.13 ALKEM LABORATORIES LTD
20.13.1 COMPANY OVERVIEW
20.13.2 REVENUE ANALYSIS
20.13.3 GEOGRAPHIC PRESENCE
20.13.4 PRODUCT PORTFOLIO
20.13.5 RECENT DEVELOPEMENTS
20.14 DAIICHI SANKYO COMPANY, LIMITED
20.14.1 COMPANY OVERVIEW
20.14.2 REVENUE ANALYSIS
20.14.3 GEOGRAPHIC PRESENCE
20.14.4 PRODUCT PORTFOLIO
20.14.5 RECENT DEVELOPEMENTS
20.15 MYLAN N.V. (VIATRIS INC.)
20.15.1 COMPANY OVERVIEW
20.15.2 REVENUE ANALYSIS
20.15.3 GEOGRAPHIC PRESENCE
20.15.4 PRODUCT PORTFOLIO
20.15.5 RECENT DEVELOPEMENTS
20.16 AUROBINDO PHARMA
20.16.1 COMPANY OVERVIEW
20.16.2 REVENUE ANALYSIS
20.16.3 GEOGRAPHIC PRESENCE
20.16.4 PRODUCT PORTFOLIO
20.16.5 RECENT DEVELOPEMENTS
20.17 ZYDUS PHARMACEUTICALS, INC.
20.17.1 COMPANY OVERVIEW
20.17.2 REVENUE ANALYSIS
20.17.3 GEOGRAPHIC PRESENCE
20.17.4 PRODUCT PORTFOLIO
20.17.5 RECENT DEVELOPEMENTS
20.18 LUPIN
20.18.1 COMPANY OVERVIEW
20.18.2 REVENUE ANALYSIS
20.18.3 GEOGRAPHIC PRESENCE
20.18.4 PRODUCT PORTFOLIO
20.18.5 RECENT DEVELOPEMENTS
20.19 SANDOZ INTERNATIONAL GMBH
20.19.1 COMPANY OVERVIEW
20.19.2 REVENUE ANALYSIS
20.19.3 GEOGRAPHIC PRESENCE
20.19.4 PRODUCT PORTFOLIO
20.19.5 RECENT DEVELOPEMENTS
20.2 FRESENIUS MEDICAL CARE AG & CO. KGAA
20.20.1 COMPANY OVERVIEW
20.20.2 REVENUE ANALYSIS
20.20.3 GEOGRAPHIC PRESENCE
20.20.4 PRODUCT PORTFOLIO
20.20.5 RECENT DEVELOPEMENTS
20.21 SANOFI
20.21.1 COMPANY OVERVIEW
20.21.2 REVENUE ANALYSIS
20.21.3 GEOGRAPHIC PRESENCE
20.21.4 PRODUCT PORTFOLIO
20.21.5 RECENT DEVELOPEMENTS
20.22 CIPLA INC.
20.22.1 COMPANY OVERVIEW
20.22.2 REVENUE ANALYSIS
20.22.3 GEOGRAPHIC PRESENCE
20.22.4 PRODUCT PORTFOLIO
20.22.5 RECENT DEVELOPEMENTS
20.23 ASTRAZENECA
20.23.1 COMPANY OVERVIEW
20.23.2 REVENUE ANALYSIS
20.23.3 GEOGRAPHIC PRESENCE
20.23.4 PRODUCT PORTFOLIO
20.23.5 RECENT DEVELOPEMENTS
20.24 BRISTOL-MYERS SQUIBB
20.24.1 COMPANY OVERVIEW
20.24.2 REVENUE ANALYSIS
20.24.3 GEOGRAPHIC PRESENCE
20.24.4 PRODUCT PORTFOLIO
20.24.5 RECENT DEVELOPEMENTS
20.25 PAR PHARMACEUTICALS
20.25.1 COMPANY OVERVIEW
20.25.2 REVENUE ANALYSIS
20.25.3 GEOGRAPHIC PRESENCE
20.25.4 PRODUCT PORTFOLIO
20.25.5 RECENT DEVELOPEMENTS
20.26 HIKMA PHARMACEUTICALS PLC
20.26.1 COMPANY OVERVIEW
20.26.2 REVENUE ANALYSIS
20.26.3 GEOGRAPHIC PRESENCE
20.26.4 PRODUCT PORTFOLIO
20.26.5 RECENT DEVELOPEMENTS
20.27 RECKITT BENCKISER
20.27.1 COMPANY OVERVIEW
20.27.2 REVENUE ANALYSIS
20.27.3 GEOGRAPHIC PRESENCE
20.27.4 PRODUCT PORTFOLIO
20.27.5 RECENT DEVELOPEMENTS
20.28 PERRIGO
20.28.1 COMPANY OVERVIEW
20.28.2 REVENUE ANALYSIS
20.28.3 GEOGRAPHIC PRESENCE
20.28.4 PRODUCT PORTFOLIO
20.28.5 RECENT DEVELOPEMENTS
20.29 TAISHO PHARMACEUTICAL
20.29.1 COMPANY OVERVIEW
20.29.2 REVENUE ANALYSIS
20.29.3 GEOGRAPHIC PRESENCE
20.29.4 PRODUCT PORTFOLIO
20.29.5 RECENT DEVELOPEMENTS
20.3 MALLINCKRODT
20.30.1 COMPANY OVERVIEW
20.30.2 REVENUE ANALYSIS
20.30.3 GEOGRAPHIC PRESENCE
20.30.4 PRODUCT PORTFOLIO
20.30.5 RECENT DEVELOPEMENTS
20.31 AMGEN, INC.
20.31.1 COMPANY OVERVIEW
20.31.2 REVENUE ANALYSIS
20.31.3 GEOGRAPHIC PRESENCE
20.31.4 PRODUCT PORTFOLIO
20.31.5 RECENT DEVELOPEMENTS
20.32 ARENA PHARMACEUTICALS, INC.
20.32.1 COMPANY OVERVIEW
20.32.2 REVENUE ANALYSIS
20.32.3 GEOGRAPHIC PRESENCE
20.32.4 PRODUCT PORTFOLIO
20.32.5 RECENT DEVELOPEMENTS
20.33 STADA ARZNEIMITTEL AG
20.33.1 COMPANY OVERVIEW
20.33.2 REVENUE ANALYSIS
20.33.3 GEOGRAPHIC PRESENCE
20.33.4 PRODUCT PORTFOLIO
20.33.5 RECENT DEVELOPEMENTS
20.34 ACCORD HEALTHCARE GMBH
20.34.1 COMPANY OVERVIEW
20.34.2 REVENUE ANALYSIS
20.34.3 GEOGRAPHIC PRESENCE
20.34.4 PRODUCT PORTFOLIO
20.34.5 RECENT DEVELOPEMENTS
20.35 ASCENDIS PHARMA GROUP
20.35.1 COMPANY OVERVIEW
20.35.2 REVENUE ANALYSIS
20.35.3 GEOGRAPHIC PRESENCE
20.35.4 PRODUCT PORTFOLIO
20.35.5 RECENT DEVELOPEMENTS
20.36 ALVOGEN
20.36.1 COMPANY OVERVIEW
20.36.2 REVENUE ANALYSIS
20.36.3 GEOGRAPHIC PRESENCE
20.36.4 PRODUCT PORTFOLIO
20.36.5 RECENT DEVELOPEMENTS
20.37 ANI PHARMACEUTICALS, INC.
20.37.1 COMPANY OVERVIEW
20.37.2 REVENUE ANALYSIS
20.37.3 GEOGRAPHIC PRESENCE
20.37.4 PRODUCT PORTFOLIO
20.37.5 RECENT DEVELOPEMENTS
20.38 ACELLA PHARMACEUTICALS, LLC
20.38.1 COMPANY OVERVIEW
20.38.2 REVENUE ANALYSIS
20.38.3 GEOGRAPHIC PRESENCE
20.38.4 PRODUCT PORTFOLIO
20.38.5 RECENT DEVELOPEMENTS
20.39 GLENMARK PHARMACEUTICALS
20.39.1 COMPANY OVERVIEW
20.39.2 REVENUE ANALYSIS
20.39.3 GEOGRAPHIC PRESENCE
20.39.4 PRODUCT PORTFOLIO
20.39.5 RECENT DEVELOPEMENTS
20.4 HORIZON THERAPEUTICS PLC
20.40.1 COMPANY OVERVIEW
20.40.2 REVENUE ANALYSIS
20.40.3 GEOGRAPHIC PRESENCE
20.40.4 PRODUCT PORTFOLIO
20.40.5 RECENT DEVELOPEMENTS
20.41 SANIS
20.41.1 COMPANY OVERVIEW
20.41.2 REVENUE ANALYSIS
20.41.3 GEOGRAPHIC PRESENCE
20.41.4 PRODUCT PORTFOLIO
20.41.5 RECENT DEVELOPEMENTS
20.42 MAYNE PHARMA
20.42.1 COMPANY OVERVIEW
20.42.2 REVENUE ANALYSIS
20.42.3 GEOGRAPHIC PRESENCE
20.42.4 PRODUCT PORTFOLIO
20.42.5 RECENT DEVELOPEMENTS
20.43 OTSUKA PHARMACEUTICALS
20.43.1 COMPANY OVERVIEW
20.43.2 REVENUE ANALYSIS
20.43.3 GEOGRAPHIC PRESENCE
20.43.4 PRODUCT PORTFOLIO
20.43.5 RECENT DEVELOPEMENTS
20.44 WACKHARDT
20.44.1 COMPANY OVERVIEW
20.44.2 REVENUE ANALYSIS
20.44.3 GEOGRAPHIC PRESENCE
20.44.4 PRODUCT PORTFOLIO
20.44.5 RECENT DEVELOPEMENTS
20.45 TORQUE PHARMACEUTICALS PVT. LTD
20.45.1 COMPANY OVERVIEW
20.45.2 REVENUE ANALYSIS
20.45.3 GEOGRAPHIC PRESENCE
20.45.4 PRODUCT PORTFOLIO
20.45.5 RECENT DEVELOPEMENTS
20.46 JIANGSU HENGRUI PHARMACEUTICALS CO., LTD.
20.46.1 COMPANY OVERVIEW
20.46.2 REVENUE ANALYSIS
20.46.3 GEOGRAPHIC PRESENCE
20.46.4 PRODUCT PORTFOLIO
20.46.5 RECENT DEVELOPEMENTS
20.47 KRKA LTD
20.47.1 COMPANY OVERVIEW
20.47.2 REVENUE ANALYSIS
20.47.3 GEOGRAPHIC PRESENCE
20.47.4 PRODUCT PORTFOLIO
20.47.5 RECENT DEVELOPEMENTS
20.48 HYPERA PHARMA
20.48.1 COMPANY OVERVIEW
20.48.2 REVENUE ANALYSIS
20.48.3 GEOGRAPHIC PRESENCE
20.48.4 PRODUCT PORTFOLIO
20.48.5 RECENT DEVELOPEMENTS
20.49 HEBEI TIANCHENG PHARMACEUTICAL COMPANY LTD
20.49.1 COMPANY OVERVIEW
20.49.2 REVENUE ANALYSIS
20.49.3 GEOGRAPHIC PRESENCE
20.49.4 PRODUCT PORTFOLIO
20.49.5 RECENT DEVELOPEMENTS
20.5 POLY PHARMACEUTICALS, INC.
20.50.1 COMPANY OVERVIEW
20.50.2 REVENUE ANALYSIS
20.50.3 GEOGRAPHIC PRESENCE
20.50.4 PRODUCT PORTFOLIO
20.50.5 RECENT DEVELOPEMENTS
21 RELATED REPORTS
22 CONCLUSION
23 QUESTIONNAIRE
24 ABOUT DATA BRIDGE MARKET RESEARCH

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。