欧洲真皮填充剂市场,按产品类型(可生物降解真皮填充剂、不可生物降解真皮填充剂)、材料类型(天然真皮填充剂、合成真皮填充剂)、应用(拉皮、隆鼻、重建手术、面部线条矫正、唇部增强、皮肤松弛、面颊凹陷、皮肤平滑、牙科、美学修复、唇部丰满、疤痕治疗、下巴增大、脂肪萎缩治疗、耳垂年轻化等)、药物类型(品牌、通用)、最终用户(皮肤科诊所、门诊手术中心、医院、学术研究机构等)、分销渠道(直接招标、药店、零售药店、网上药店等)划分——行业趋势和预测到 2029 年。
欧洲皮肤填充剂市场分析与洞察
真皮填充剂是一种凝胶状物质,注射到皮肤下可恢复失去的体积,通常可消除皱纹和平滑细纹或增强面部轮廓。近年来,真皮填充剂的使用量迅速增长,因为各种产品都可用于增强美观和恢复活力,而这些以前只能通过手术来实现。这些产品已成为非常流行的面部恢复方法。作为微创产品,真皮填充剂在美容体积替代疗法中可立即见效。这些设备可增加面部体积,改善和提高皮肤质量。各种方法(例如注射)都可用于增大。这些填充剂可使嘴唇更丰满、更丰满,以达到美容目的。有许多类型的真皮填充剂可注射到嘴唇和嘴周围,根据所用产品的类型,可暂时或永久保持嘴唇的体积。一些市场参与者参与了新产品和创新产品,他们的产品正在筹备中。过去几年,为了促进真皮填充剂市场的增长,市场参与者开发了新的创新真皮填充剂产品,并不断增强其产品组合。许多市场参与者都参与了真皮填充剂的生产,并通过创新为市场增长铺平了道路。
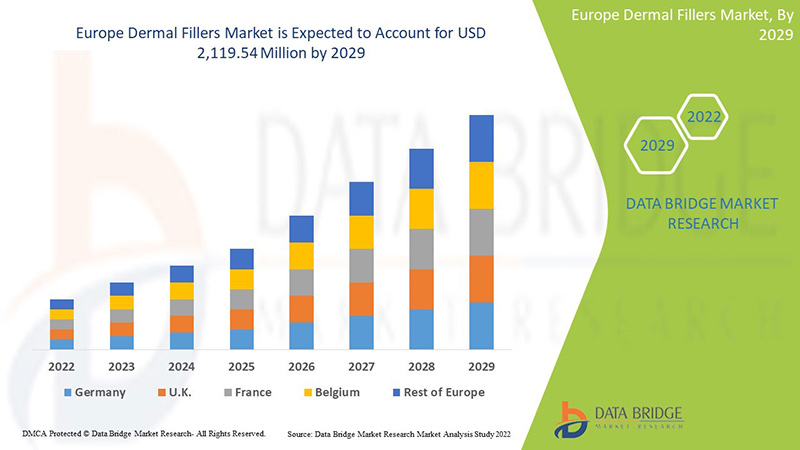
Data Bridge Market Research 分析,真皮填充剂市场预计到 2029 年将达到 21.1954 亿美元的价值,在 2022-2029 年预测期内的复合年增长率为 10.0%。
|
报告指标 |
细节 |
|
预测期 |
2022 至 2029 年 |
|
基准年 |
2021 |
|
历史年份 |
2020 (可定制为 2019-2014) |
|
定量单位 |
收入(百万美元)、销量(单位)、定价(美元) |
|
涵盖的领域 |
按产品类型(可生物降解的真皮填充剂、不可生物降解的真皮填充剂)、材料类型(天然真皮填充剂、合成真皮填充剂)、应用(拉皮、隆鼻、重建手术、面部线条矫正、唇部增强、皮肤松弛、面颊凹陷、皮肤平滑、牙科、美容修复、丰唇、疤痕治疗、下巴增大、脂肪萎缩治疗、耳垂年轻化等)、药品类型(品牌、通用)、最终用户(皮肤科诊所、门诊手术中心、医院、学术研究机构等)、分销渠道(直接招标、药店、零售药店、网上药店等) |
|
覆盖国家 |
德国、意大利、法国、英国、西班牙、俄罗斯、荷兰、瑞士、比利时、土耳其和欧洲其他地区 |
|
涵盖的市场参与者 |
Allergan(Abbvie 公司子公司)、Prollenium Medical Technologies、Suneva Medical、Revance Therapeutics, Inc.、FillMed Laboratories、Anika Therapeutics, Inc、Ipsen Pharma、BIOXIS Pharmaceuticals、Zimmer Aesthetic、浙江景嘉医疗科技有限公司、Medytox、Contura International ltd.、上海瑞阳医疗科技有限公司、Humedix(HUONS GLOBAL 子公司)、Galderma Laboratories, LP、Merz North America, Inc.(Merz Pharma 子公司)、Croma-Pharma GmbH、Sinclair Pharma(华东医药股份有限公司子公司)、Teoxane、BioPlus Co., Ltd.、Amalian、Givaudan、Mesoestetic、Sosum Global、DSM、IBSA Nordic ApS 等。 |
市场定义
真皮填充剂是注射到皮肤中以增加丰满度和体积的物质。真皮填充剂中使用的物质包括羟基磷灰石钙(骨骼中发现的矿物质类化合物)、透明质酸、聚烷基酰亚胺、聚乳酸、聚甲基丙烯酸甲酯微球 (PMMA)。真皮填充剂可根据多种标准进行分类,包括深层真皮、植入深度(浅层、中层和上层以及皮下层);矫正的持久性(暂时性和永久性);过敏性、药剂的成分(同种异体移植、半/全合成、异种移植或自体);以及刺激行为(内源性组织增殖的生理过程)与替代填充剂(空间替代效应)。
HA 和胶原蛋白等临时真皮填充剂可生物降解,可持续 4 至 9 个月。预期的副作用和不满意也是短暂的。因此,临时填充剂始终被用作第一线治疗方法,以节省长期填充剂以供患者将来就诊。
永久性填充剂主要用于改变皮肤深层皱纹和沟纹,这些皱纹和沟纹超出了正常的面部皱纹。它们被认为是面部年轻化的绝佳选择,尤其是对于 HIV 脂肪营养不良症。聚甲基丙烯酸甲酯 (PMMA) 主要用于安全、有效和持久的效果。
皮肤填充剂市场动态
本节旨在了解市场驱动因素、优势、机遇、限制和挑战。以下内容将详细讨论所有这些内容:
驱动程序
- 微创手术日益普及
与传统的美容和整容手术方法(包括激光和其他基于能量的设备)相比,微创手术的使用已经发生了变化。对于手术或非手术程序的使用,已经开发了专门设计的用于微创手术的仪器。这些抗衰老设备有助于通过恢复和收紧皮肤来减少皮肤老化的视觉效果,从而使皮肤看起来更年轻。
微创手术是在望远镜和专门配备的手术器械的帮助下进行的手术。它针对面部不规则,包括皱纹和细纹,减少体积和轮廓以及多余脂肪。这些手术基本上没有严重不良事件的风险,因为手术不会造成切口或切口很小,恢复时间较短,这增加了对微创手术的需求。用于紧致皮肤、减少皱纹、面部轮廓和皮肤年轻化的微创能量治疗在世界范围内需求量很大。推动这些手术需求的其他原因是老龄化人口增加和医疗设施需求增加,这可以进一步减轻医疗设施的负担。
随着技术进步和通信技术的进步,人们越来越了解在医疗保健领域表现良好的美容设备和程序,这将对即将到来的时代采用微创手术产生积极影响。因此,微创手术的日益普及预计将推动真皮填充剂市场的增长。
- 老年人口不断增加
老年人口不断增加,寿命越来越长,据报道,皮肤老化问题日益严重。世界人口老龄化速度呈指数级增长,而欧洲国家具有某些共同的文化、社会和经济特征,有着类似的愿望。随着人们开始衰老,外表变得越来越年轻,这最终引起了人们对美容手术的兴趣。
中东国家老年人口比例的不断提高及其医疗保健状况的改善为患者带来了更好的治疗结果服务。
机会
- 增加对美学研究的资助活动
整形手术、去除多余毛发、紧致肌肤、抗衰老、去除多余脂肪、塑身和其他一些美容手术都是通过微创手术进行的,这些手术是在医疗美容设备下进行的,用于改善外观、美化和改善身体的其他部位。各种基金会和政府组织都在美学研究方面投入了大量资金。
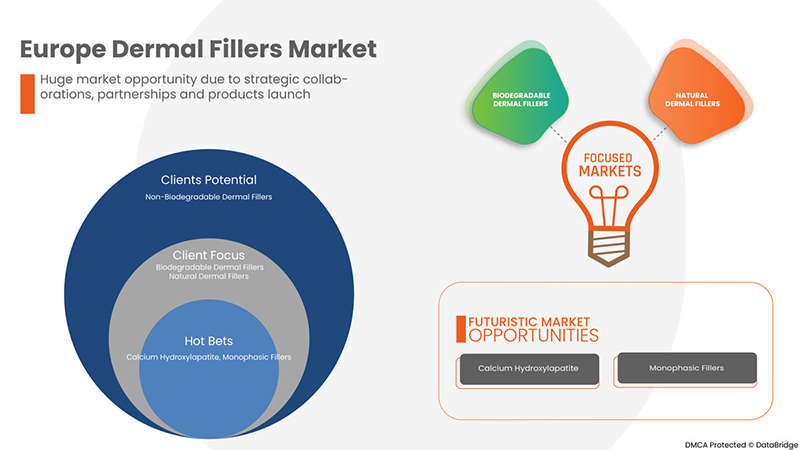
- 新型真皮填充剂的进展
真皮填充剂是凝胶状物质,注射到皮下可恢复流失的体积、软化皱纹和抚平细纹。它也用于改善面部轮廓;每年有超过 100 万人选择这种流行的面部改造疗法来恢复皱纹和面部线条的外观。真皮填充剂是一种有效的治疗方法,可让您看起来更年轻,无需恢复期或手术。真皮填充剂药物注射到皮肤中,有助于填充面部皱纹。有不同类型的真皮填充剂药物可供选择,最常见的类型是羟基磷灰石钙、透明质酸、聚烷基酰亚胺、聚乳酸等。真皮填充剂品牌的进步将有助于促进市场需求。
限制/挑战
- 美容手术费用高昂
成本始终是选择性手术的一个考虑因素。真皮填充剂的成本取决于填充剂的类型和治疗中使用的量。此外,治疗费用取决于进行真皮填充剂治疗的人员的资质和专业知识。真皮填充剂的美容治疗是一种安全的门诊手术,是一种非常流行的恢复体积损失和治疗某些衰老迹象的治疗方法,但手术的高成本预计将阻碍市场的需求
- 缺乏熟练的专业人员
美容治疗程序有多种类型,包括基于激光的技术、基于能量的技术以及强脉冲光等。所有这些技术都需要合格的人际交往技巧才能进行有效的治疗。
此外,该领域的技术快速进步也导致专业技能的缺乏。缺乏专业技能的人员对设备操作和手术程序的执行构成重大挑战。
最新动态
- 2022 年 4 月,Sinclair Pharma 宣布该公司已获得 Perfectha Lidocaine 在皱纹矫正、面部轮廓和体积恢复治疗方面的欧洲 CE 标志。此 CE 认证使 Perfectha Lidocaine 在英国和所有主要欧洲市场上市。
皮肤填充剂市场细分
真皮填充剂市场根据产品类型、材料类型、应用、药物类型、最终用户和分销渠道进行细分。细分市场之间的增长有助于您分析利基增长领域和进入市场的策略,并确定您的核心应用领域和目标市场的差异。
按产品类型
- 可生物降解的真皮填充剂
- 不可生物降解的皮肤填充剂
根据产品类型,真皮填充剂市场分为可生物降解的真皮填充剂和不可生物降解的真皮填充剂。
按材料类型
- 天然皮肤填充剂
- 合成皮肤填充剂
根据材料类型,市场分为天然真皮填充剂和合成真皮填充剂。
按应用
- 面部拉皮
- 鼻整形手术
- 重建手术
- 面部线条矫正
- 丰唇
- 皮肤松弛
- 面颊凹陷
- 皮肤光滑
- 牙科
- 美容修复
- 唇梅
- 疤痕治疗
- 下巴整形
- 脂肪萎缩治疗
- 耳垂复原
- 其他的
根据应用,市场细分为面部提升、鼻整形、重建手术、面部线条矫正、唇部增强、皮肤松弛、脸颊凹陷、皮肤平滑、牙科、美容修复、疤痕治疗、下巴增大、脂肪萎缩治疗、耳垂年轻化等。
按药物类型
- 品牌
- 通用的
根据药品类型,市场分为品牌药品和仿制药。
按最终用户
- 皮肤科诊所
- 医院
- 门诊手术中心
- 学术研究机构
- 其他的
根据最终用户,市场分为皮肤病诊所、门诊手术中心、医院、学术研究机构和其他。
按分销渠道
- 直接招标
- 药店
- 零售药店
- 网上药店
- 其他的
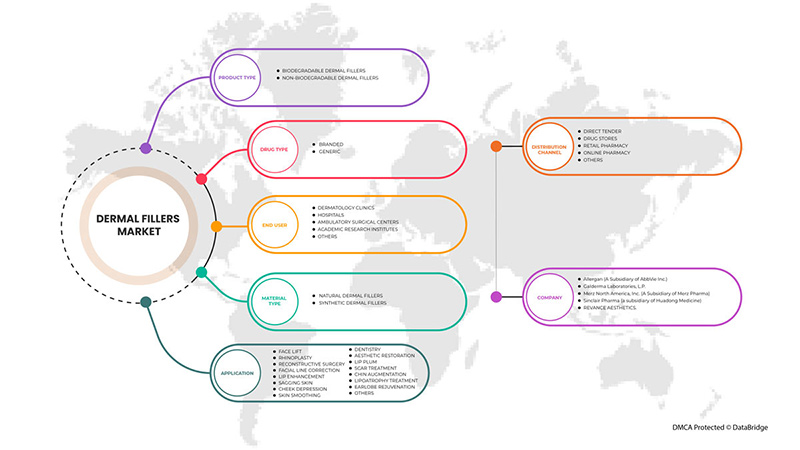
根据分销渠道,市场分为直接招标、药店、零售药店、网上药店和其他。
真皮填充剂市场区域分析/见解
对真皮填充剂市场进行了分析,并按国家、产品类型、材料类型、应用、药物类型、最终用户和分销渠道提供了市场规模洞察和趋势。
真皮填充剂市场报告涉及的国家包括德国、意大利、法国、英国、西班牙、俄罗斯、荷兰、瑞士、比利时、土耳其和欧洲其他地区。
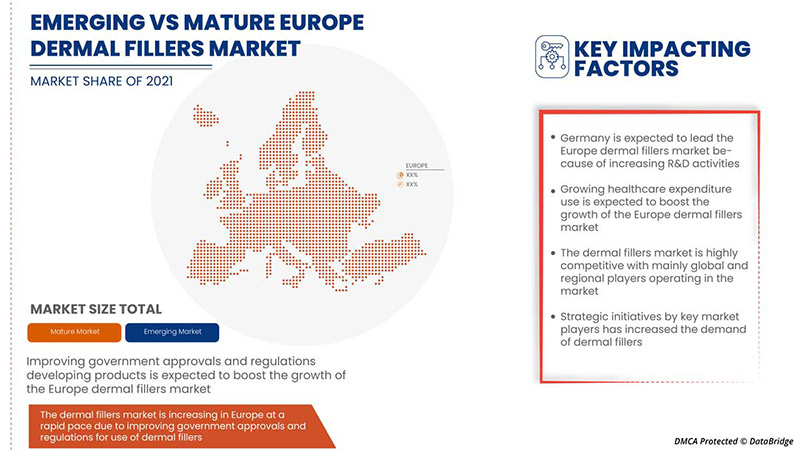
由于皮肤填充设备技术的不断提高和可靠性的不断提高,德国有望占据市场主导地位。
报告的国家部分还提供了影响市场当前和未来趋势的各个市场影响因素和市场监管变化。下游和上游价值链分析、技术趋势和波特五力分析、案例研究等数据点是用于预测各个国家市场情景的一些指标。此外,在提供国家数据的预测分析时,还考虑了品牌的存在和可用性以及由于来自本地和国内品牌的大量或稀缺竞争而面临的挑战、国内关税和贸易路线的影响。
竞争格局和真皮填充剂市场份额分析
真皮填充剂市场竞争格局按竞争对手提供详细信息。详细信息包括公司概况、公司财务状况、收入、市场潜力、研发投资、新市场计划、全球影响力、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度、应用主导地位。以上提供的数据点仅与公司对真皮填充剂市场的关注有关。
市场上的一些主要参与者包括 Allergan(Abbvie 公司旗下的子公司)、Prollenium Medical Technologies、Suneva Medical、Revance Therapeutics 公司、FillMed Laboratories、Anika Therapeutics 公司、Ipsen Pharma、BIOXIS Pharmaceuticals、Zimmer Aesthetic、浙江景嘉医疗科技有限公司、Medytox、Contura International ltd.、上海瑞阳医疗科技有限公司、Humedix(HUONS GLOBAL 旗下的子公司)、Galderma Laboratories,LP、Merz North America,Inc.(Merz Pharma 旗下的子公司)、Croma-Pharma GmbH、Sinclair Pharma(华东医药股份有限公司旗下的子公司)、Teoxane、BioPlus Co.,Ltd.、Amalian、Givaudan、Mesoestetic、Sosum Global、DSM、IBSA Nordic ApS 等。
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE DERMAL FILLERS MARKET
1.4 LIMITATION
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCTS LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 EPIDEMIOLOGY PROCEDURES PER COUNTRY
5 REGULATIONS OF EUROPE DERMAL FILLERS MARKET
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF MINIMALLY INVASIVE PROCEDURES
6.1.2 INCREASING GERIATRIC POPULATION
6.1.3 TECHNOLOGICAL ADVANCEMENTS IN DERMAL FILLERS
6.1.4 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.2 RESTRAINTS
6.2.1 HIGH COST OF AESTHETIC PROCEDURES
6.2.2 ESCALATING PRODUCT RECALL
6.2.3 FDA GUIDELINES FOR ADVANCED DERMAL FILLER INJECTORS
6.3 OPPORTUNITIES
6.3.1 INCREASING HEALTHCARE EXPENDITURE
6.3.2 INCREASING FUNDING ACTIVITIES FOR AESTHETIC RESEARCH
6.3.3 ADVANCEMENT IN THE NEW DERMAL FILLERS
6.3.4 INCREASING COMPENSATION AND ACCIDENTAL CLAIMS FOR DERMAL FILLER
6.4 CHALLENGES
6.4.1 LACK OF SKILLED PROFESSIONALS
6.4.2 AVAILABILITY OF ALTERNATIVES
7 EUROPE DERMAL FILLERS MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 BIODEGRADABLE DERMAL FILLERS
7.2.1 TEMPORARY BIODEGRADABLE
7.2.2 SEMI-PERMANENT BIODEGRADABLE
7.3 NON-BIODEGRADABLE DERMAL FILLERS
8 EUROPE DERMAL FILLERS MARKET, BY MATERIAL TYPE
8.1 OVERVIEW
8.2 NATURAL DERMAL FILLERS
8.2.1 HYALURONIC ACID
8.2.1.1 BY TYPE
8.2.1.1.1 MONOPHASIC FILLERS
8.2.1.2 BY TYPE
8.2.1.2.1 MONODENSIFIED
8.2.1.2.2 POLYDENSIFIED
8.2.1.2.3 BIPHASIC FILLERS
8.2.1.3 BY MATERIAL TYPE
8.2.1.3.1 SINGLE-PHASE
8.2.1.3.2 DUPLEX-PHASE
8.2.1.4 BY APPLICATION
8.2.1.4.1 FACE LIFT
8.2.1.4.2 RHINOPLASTY
8.2.1.4.3 RECONSTRUCTIVE SURGERY
8.2.1.4.4 FACIAL LINE CORRECTION
8.2.1.4.5 LIP ENHANCEMENT
8.2.1.4.6 SAGGING SKIN
8.2.1.4.7 CHEEK DEPRESSION
8.2.1.4.8 SKIN SMOOTHING
8.2.1.4.9 DENTISTRY
8.2.1.4.10 AESTHETIC RESTORATION
8.2.1.4.11 SCAR TREATMENT
8.2.1.4.12 CHIN AUGMENTATION
8.2.1.4.13 LIPOATROPHY TREATMENT
8.2.1.4.14 EARLOBE REJUVENATION
8.2.1.4.15 OTHERS
8.2.2 FAT
8.2.2.1 BY APPLICATION
8.2.2.1.1 FACE LIFT
8.2.2.1.2 RHINOPLASTY
8.2.2.1.3 RECONSTRUCTIVE SURGERY
8.2.2.1.4 FACIAL LINE CORRECTION
8.2.2.1.5 LIP ENHANCEMENT
8.2.2.1.6 SAGGING SKIN
8.2.2.1.7 CHEEK DEPRESSION
8.2.2.1.8 SKIN SMOOTHING
8.2.2.1.9 DENTISTRY
8.2.2.1.10 AESTHETIC RESTORATION
8.2.2.1.11 SCAR TREATMENT
8.2.2.1.12 CHIN AUGMENTATION
8.2.2.1.13 LIPOATROPHY TREATMENT
8.2.2.1.14 EARLOBE REJUVENATION
8.2.2.1.15 OTHERS
8.2.3 COLLAGEN
8.2.3.1 BY APPLICATION
8.2.3.1.1 FACE LIFT
8.2.3.1.2 RHINOPLASTY
8.2.3.1.3 RECONSTRUCTIVE SURGERY
8.2.3.1.4 FACIAL LINE CORRECTION
8.2.3.1.5 LIP ENHANCEMENT
8.2.3.1.6 SAGGING SKIN
8.2.3.1.7 CHEEK DEPRESSION
8.2.3.1.8 SKIN SMOOTHING
8.2.3.1.9 DENTISTRY
8.2.3.1.10 AESTHETIC RESTORATION
8.2.3.1.11 SCAR TREATMENT
8.2.3.1.12 CHIN AUGMENTATION
8.2.3.1.13 LIPOATROPHY TREATMENT
8.2.3.1.14 EARLOBE REJUVENATION
8.2.3.1.15 OTHERS
8.2.4 OTHERS
8.3 SYNTHETIC DERMAL FILLERS
8.3.1 POLY-L-LACTIC ACID
8.3.1.1 BY APPLICATION
8.3.1.1.1 FACE LIFT
8.3.1.1.2 RHINOPLASTY
8.3.1.1.3 RECONSTRUCTIVE SURGERY
8.3.1.1.4 FACIAL LINE CORRECTION
8.3.1.1.5 LIP ENHANCEMENT
8.3.1.1.6 SAGGING SKIN
8.3.1.1.7 CHEEK DEPRESSION
8.3.1.1.8 SKIN SMOOTHING
8.3.1.1.9 DENTISTRY
8.3.1.1.10 AESTHETIC RESTORATION
8.3.1.1.11 SCAR TREATMENT
8.3.1.1.12 CHIN AUGMENTATION
8.3.1.1.13 LIPOATROPHY TREATMENT
8.3.1.1.14 EARLOBE REJUVENATION
8.3.1.1.15 OTHERS
8.3.2 CALCIUM HYDROXYLAPATITE
8.3.2.1 BY APPLICATION
8.3.2.1.1 FACE LIFT
8.3.2.1.2 RHINOPLASTY
8.3.2.1.3 RECONSTRUCTIVE SURGERY
8.3.2.1.4 FACIAL LINE CORRECTION
8.3.2.1.5 LIP ENHANCEMENT
8.3.2.1.6 SAGGING SKIN
8.3.2.1.7 CHEEK DEPRESSION
8.3.2.1.8 SKIN SMOOTHING
8.3.2.1.9 DENTISTRY
8.3.2.1.10 AESTHETIC RESTORATION
8.3.2.1.11 SCAR TREATMENT
8.3.2.1.12 CHIN AUGMENTATION
8.3.2.1.13 LIPOATROPHY TREATMENT
8.3.2.1.14 EARLOBE REJUVENATION
8.3.2.1.15 OTHERS
8.3.3 POLYMETHYL-METHACRYLATE MICROSPHERES (PMMA)
8.3.3.1 BY APPLICATION
8.3.3.1.1 FACE LIFT
8.3.3.1.2 RHINOPLASTY
8.3.3.1.3 RECONSTRUCTIVE SURGERY
8.3.3.1.4 FACIAL LINE CORRECTION
8.3.3.1.5 LIP ENHANCEMENT
8.3.3.1.6 SAGGING SKIN
8.3.3.1.7 CHEEK DEPRESSION
8.3.3.1.8 SKIN SMOOTHING
8.3.3.1.9 DENTISTRY
8.3.3.1.10 AESTHETIC RESTORATION
8.3.3.1.11 SCAR TREATMENT
8.3.3.1.12 CHIN AUGMENTATION
8.3.3.1.13 LIPOATROPHY TREATMENT
8.3.3.1.14 EARLOBE REJUVENATION
8.3.3.1.15 OTHERS
8.3.4 POLYALKYLIMIDE
8.3.4.1 BY APPLICATION
8.3.4.1.1 FACE LIFT
8.3.4.1.2 RHINOPLASTY
8.3.4.1.3 RECONSTRUCTIVE SURGERY
8.3.4.1.4 FACIAL LINE CORRECTION
8.3.4.1.5 LIP ENHANCEMENT
8.3.4.1.6 SAGGING SKIN
8.3.4.1.7 CHEEK DEPRESSION
8.3.4.1.8 SKIN SMOOTHING
8.3.4.1.9 DENTISTRY
8.3.4.1.10 AESTHETIC RESTORATION
8.3.4.1.11 SCAR TREATMENT
8.3.4.1.12 CHIN AUGMENTATION
8.3.4.1.13 LIPOATROPHY TREATMENT
8.3.4.1.14 EARLOBE REJUVENATION
8.3.4.1.15 OTHERS
8.3.5 OTHERS
9 EUROPE DERMAL FILLERS MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 FACE LIFT
9.2.1 DEEP PLANE/SMAS FACE
9.2.2 MINI FACE LIFT
9.2.3 MID-FACE LIFT
9.2.4 JAW LINE
9.2.5 CUTANEOUS LIFT
9.2.6 TEMPORAL OR BROW LIFT
9.2.7 LIQUID FACE LIFT
9.2.8 OTHERS
9.2.8.1 JUVEDERM
9.2.8.2 RESTYLANE
9.2.8.3 SCULPTRA
9.2.8.4 DYSPORT
9.2.8.5 OTHERS
9.3 RHINOPLASTY
9.3.1 JUVEDERM
9.3.1.1 JUVEDERM XC
9.3.1.2 JUVEDERM ULTRA XC
9.3.1.3 JUVEDERM VOLUMA
9.3.1.4 JUVEDERM VOLBELLA
9.3.1.5 JUVEDERM VOLLURE XC
9.3.2 RESTYLANE
9.3.2.1 RESTYLANE SILK
9.3.2.2 RESTYLANE LYFT
9.3.2.3 RESTYLANE REFYNE
9.3.2.4 RESTYLANE DEFYNE
9.3.2.5 RESTYLANE-L
9.3.3 OTHERS
9.4 RECONSTRUCTIVE SURGERY
9.4.1 JUVEDERM
9.4.2 RESTYLANE
9.4.3 OTHERS
9.5 FACIAL LINE CORRECTION
9.5.1 DYNAMIC WRINKLES
9.5.2 STATIC WRINKLES
9.5.3 WRINKLE FOLDS
9.5.3.1 FOREHEAD LINES
9.5.3.2 WORRY LINES
9.5.3.3 BUNNIES
9.5.3.4 CROW’S FEET
9.5.3.5 LAUGH LINES
9.5.3.6 LIP LINES
9.5.3.7 MARIONETTE LINES
9.5.3.8 OTHERS
9.5.3.8.1 JUVEDERM
9.5.3.8.2 RESTYLANE
9.5.3.8.3 RADIESSE
9.5.3.8.4 BELOTERO
9.5.3.8.5 OTHERS
9.6 LIP ENHANCEMENT
9.6.1 JUVEDERM
9.6.1.1 JUVÉDERM XC
9.6.1.2 JUVÉDERM ULTRA XC
9.6.1.3 JUVÉDERM VOLUMA
9.6.1.4 JUVÉDERM VOLBELLA
9.6.1.5 JUVÉDERM VOLLURE XC
9.6.2 RESTYLANE
9.6.2.1 RESTYLANE SILK
9.6.2.2 RESTYLANE LYFT
9.6.2.3 RESTYLANE REFYNE
9.6.2.4 RESTYLANE DEFYNE
9.6.2.5 RESTYLANE-L
9.6.3 BELOTERO BALANCE
9.6.4 REVANESSE VERSA
9.6.5 HYLAFORM
9.6.6 PREVELLE SILK
9.6.7 OTHERS
9.7 SAGGING SKIN
9.7.1 JUVEDERM
9.7.2 RESTYLANE
9.7.3 BELOTERO
9.7.4 OTHERS
9.8 CHEEK DEPRESSION
9.8.1 JUVEDERM VOLUMA
9.8.2 RESTYLANE-LYFT
9.8.3 SCULPTRA
9.8.4 RADIESSE
9.8.5 OTHERS
9.9 SKIN SMOOTHENING
9.9.1 RESTYLANE
9.9.2 BELOTERO
9.9.3 BELLAFIL
9.9.4 OTHERS
9.1 DENTISTRY
9.10.1 JUVEDERM
9.10.2 RESTYLANE
9.10.3 RADIESSE
9.10.4 OTHERS
9.11 AESTHETIC RESTORATION
9.11.1 JUVEDERM
9.11.1.1 JUVEDERM ULTRA XC
9.11.1.2 JUVEDERM VOLLURE XC
9.11.1.3 JUVEDERM VOLBELLA XC
9.11.2 RESTYLANE
9.11.2.1 RESTYLANE-L
9.11.2.2 RESTYLANE SILK
9.11.2.3 RESTYLANE REFYNE AND DEFYNE
9.11.2.4 RESTYLANE LYFT
9.12 REVANESSE VERSA
9.13 SCULPTRA
9.14 RHA
9.14.1 RHA 2
9.14.2 RHA 3
9.14.3 RHA 4
9.15 BELLAFIL
9.16 BELOTERO BALANCE
9.17 OTHERS
9.18 LIP PLUM
9.18.1 RESTYLANE
9.18.2 BELOTERO
9.18.3 OTHERS
9.19 SCAR TREATMENT
9.19.1 JUVEDERM
9.19.2 RESTYLANE
9.19.3 RADIESSE
9.19.4 BELOTERO
9.19.5 PERLANE
9.19.6 OTHERS
9.19.6.1 KELOID SCARS
9.19.6.2 CONTRACTURE SCARS
9.19.6.3 HYPERTROPHIC SCARS
9.19.6.4 ACNE SCARS
9.19.6.5 OTHERS
9.2 CHIN AUGMENTATION
9.20.1 JUVEDERM VOLUMA XC
9.20.2 RESTYLANE
9.20.3 OTHERS
9.21 LIPOATROPHY TREATMENT
9.21.1 SCULPTRA
9.21.2 OTHERS
9.22 EARLOBE REJUVENATION
9.22.1 JUVEDERM
9.22.2 RESTYLANE
9.22.3 SCULPTRA
9.22.4 BELOTERO
9.22.5 ELLANSE
9.22.6 OTHERS
9.23 OTHERS
10 EUROPE DERMAL FILLERS MARKET, BY DRUG TYPE
10.1 OVERVIEW
10.2 BRANDED
10.2.1 JUVEDERM
10.2.1.1 JUVÉDERM XC
10.2.1.2 JUVÉDERM ULTRA XC
10.2.1.3 JUVÉDERM ULTRA PLUS XC
10.2.1.4 JUVÉDERM VOLBELLA
10.2.1.5 JUVÉDERM VOLUMA
10.2.1.6 JUVÉDERM VOLLURE
10.2.2 RESTYLANE
10.2.2.1 RESTYLANE-L
10.2.2.2 RESTYLANE REFYNE
10.2.2.3 RESTYLANE DEFYNE
10.2.2.4 RESTYLANE LYFT
10.2.2.5 RESTYLANE SILK
10.2.2.6 RESTYLANE KYSSE
10.2.2.7 RESTYLANE CONTOUR
10.2.3 RADIESSE
10.2.4 SCULPTRA
10.2.5 ELLANSE
10.2.5.1 ELLANSE-S
10.2.5.2 ELLANSE-M
10.2.5.3 ELLANSE-L
10.2.5.4 ELLANSE-E
10.2.6 BELLAFILL
10.2.7 AQUAMID
10.2.8 OTHERS
10.3 GENERIC
11 EUROPE DERMAL FILLERS MARKET, BY END USER
11.1 OVERVIEW
11.2 DERMATOLOGY CLINICS
11.3 HOSPITALS
11.4 AMBULATORY SURGICAL CENTERS
11.5 ACADEMIC RESEARCH INSTITUTES
11.6 OTHERS
12 EUROPE DERMAL FILLERS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 DRUG STORES
12.4 RETAIL PHARMACY
12.5 ONLINE PHARMACY
12.6 OTHERS
13 EUROPE DERMAL FILLERS MARKET, BY GEOGRAPHY
13.1 EUROPE
13.1.1 GERMANY
13.1.2 ITALY
13.1.3 FRANCE
13.1.4 U.K.
13.1.5 SPAIN
13.1.6 RUSSIA
13.1.7 NETHERLANDS
13.1.8 SWITZERLAND
13.1.9 BELGIUM
13.1.10 TURKEY
13.1.11 REST OF EUROPE
14 EUROPE DERMA FILLERS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ALLERGAN (A SUBSIDIARY OF ABBVIE INC.)
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 GALDERMA LABORATORIES, L.P
16.2.1 COMPANY SNAPSHOT
16.2.2 COMPANY SHARE ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENTS
16.3 MERZ NORTH AMERICA, INC (A SUBSIDIARY OF MERZ PHARMA)
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENTS
16.4 SINCLAIR PHARMA (A SUBSIDIARY OF HUADONG MEDICINE)
16.4.1 COMPANY SNAPSHOT
16.4.2 COMPANY SHARE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENT
16.5 REVANCE THERAPEUTICS, INC.
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 AMALIAN
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENTS
16.7 ANIKA THERAPEUTICS, INC.
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENTS
16.8 BIOPLUS CO., LTD.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 BIOXIS PHARMACEUTICALS
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENTS
16.1 CONTURA INTERNATIONAL LTD.
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 CROMA-PHARMA GMBH
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENT
16.12 DSM
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 FILLMED
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 GIVAUDAN
16.14.1 COMPANY SNAPSHOT
16.14.2 RECENT FINANCIALS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 HUMEDIX (A SUBSIDIARY OF HUONS EUROPE)
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 IBSA FARMACEUTICI ITALIA SRL (A SUBSIDIARY OF IBSA INSTITUT BIOCHIMIQUE SA)
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENT
16.17 IPSEN PHARMA.
16.17.1 COMPANY SNAPSHOT
16.17.2 REVENUE ANALYSIS
16.17.3 PRODUCT PORTFOLIO
16.17.4 RECENT DEVELOPMENT
16.18 MEDYTROX
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 MESOESTETIC
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENTS
16.2 PROLLENIUM MEDICAL TECHNOLOGIES
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENT
16.21 SHANGHAI REYOUNGEL MEDICAL TECHNOLOGY COMPANY LIMITED
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENTS
16.22 SOSUM EUROPE
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENT
16.23 SUNEVA MEDICAL
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENT
16.24 TEOXANE SA
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENTS
16.25 ZHEJIANG JINGJIA MEDICAL TECHNOLOGY CO., LTD
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENT
16.26 ZIMMER AESTHETICS
16.26.1 COMPANY SNAPSHOT
16.26.2 PRODUCT PORTFOLIO
16.26.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
表格列表
TABLE 1 COST OF DERMAL FILLERS
TABLE 2 COST OF PROCEDURE
TABLE 3 ASAPS PROCEDURE FACTS
TABLE 4 COMPENSATION COST FOR DERMAL FILLERS
TABLE 5 EUROPE DERMAL FILLERS MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 6 EUROPE DERMAL FILLERS MARKET, BY PRODUCT TYPE, VOLUME, 2020-2029 (UNITS)
TABLE 7 EUROPE BIODEGRADABLE DERMAL FILLERS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 EUROPE BIODEGRADABLE DERMAL FILLERS IN DERMAL FILLERS MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 9 EUROPE BIODEGRADABLE DERMAL FILLERS IN DERMAL FILLERS MARKET, BY PRODUCT TYPE, VOLUME, 2020-2029 (UNITS)
TABLE 10 EUROPE NON-BIODEGRADABLE DERMAL FILLERS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE DERMAL FILLERS MARKET, BY MATERIAL TYPE, 2020-2029 (USD MILLION)
TABLE 12 EUROPE NATURAL DERMAL FILLERS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE NATURAL DERMAL FILLERS IN DERMAL FILLERS MARKET, BY MATERIAL TYPE, 2020-2029 (USD MILLION)
TABLE 14 EUROPE HYALURONIC ACID IN DERMAL FILLERS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 15 EUROPE MONOPHASIC FILLERS IN DERMAL FILLERS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 16 EUROPE HYALURONIC ACID IN DERMAL FILLERS MARKET, BY MATERIAL TYPE, 2020-2029 (USD MILLION)
TABLE 17 EUROPE HYALURONIC ACID IN DERMAL FILLERS MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE FAT IN DERMAL FILLERS MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE COLLAGEN IN DERMAL FILLERS MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
TABLE 20 EUROPE SYNTHETIC DERMAL FILLERS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE SYNTHETIC DERMAL FILLERS IN DERMAL FILLERS MARKET, BY MATERIAL TYPE, 2020-2029 (USD MILLION)
TABLE 22 EUROPE POLY-L-LACTIC ACID IN DERMAL FILLERS MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE CALCIUM HYDROXYLAPATITE IN DERMAL FILLERS MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE POLYMETHYL-METHACRYLATE MICROSPHERES (PMMA) IN DERMAL FILLERS MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE POLYALKYLIMIDE IN DERMAL FILLERS MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE DERMAL FILLERS MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE FACE LIFT IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE FACE LIFT IN DERMAL FILLERS MARKET, BY TYPE, 2020- 2029 (USD MILLION)
TABLE 29 EUROPE FACE LIFT IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 30 EUROPE RHINOPLASTY IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE RHINOPLASTY IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 32 EUROPE JUVEDERM IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 33 EUROPE RESTYLANE IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 34 EUROPE RECONSTRUCTIVE SURGERY IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE RECONSTRUCTIVE SURGERY IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 36 EUROPE FACIAL LINE CORRECTION IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 EUROPE FACIAL LINE CORRECTION IN DERMAL FILLERS MARKET, BY TYPE, 2020- 2029 (USD MILLION)
TABLE 38 EUROPE FACIAL LINE CORRECTION IN DERMAL FILLERS MARKET, BY TYPE, 2020- 2029 (USD MILLION)
TABLE 39 EUROPE FACIAL LINE CORRECTION IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 40 EUROPE LIP ENHANCEMENT IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 EUROPE LIP ENHANCEMENT IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 42 EUROPE JUVEDERM IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 43 EUROPE RESTYLANE IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 44 EUROPE SAGGING SKIN IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 45 EUROPE SAGGING SKIN IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 46 EUROPE CHEEK DEPRESSION IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 EUROPE CHEEK DEPRESSION IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 48 EUROPE SKIN SMOOTHENING IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 49 EUROPE SKIN SMOOTHENING IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 50 EUROPE DENTISTRY IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 EUROPE DENTISTRY IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 52 EUROPE AESTHETIC RESTORATION IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 EUROPE AESTHETIC RESTORATION IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 54 EUROPE JUVEDERM IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 55 EUROPE RESTYLANE IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 56 EUROPE RHA IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 57 EUROPE LIP PLUM IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 58 EUROPE LIP PLUM IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 59 EUROPE SCAR TREATMENT IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 60 EUROPE SCAR TREATMENT IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 61 EUROPE SCAR TREATMENT IN DERMAL FILLERS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 62 EUROPE CHIN AUGMENTATION IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 63 EUROPE CHIN AUGMENTATION IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 64 EUROPE LIPOATROPHY TREATMENT IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 65 EUROPE LIPOATROPHY TREATMENT IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 66 EUROPE EARLOBE REJUVENTION IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 67 EUROPE EARLOBE REJUVENTION IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 68 EUROPE OTHERS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 69 EUROPE DERMAL FILLERS MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 70 EUROPE BRANDED IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 71 EUROPE BRANDED IN DERMAL FILLERS MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 72 EUROPE JUVEDERM IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 73 EUROPE RESTYLANE IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 74 EUROPE ELLANSE IN DERMAL FILLERS MARKET, BY BRAND, 2020-2029 (USD MILLION)
TABLE 75 EUROPE GENERIC IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 76 EUROPE DERMAL FILLERS MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 77 EUROPE DERMATOLOGY CLINICS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 78 EUROPE HOSPITALS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 79 EUROPE AMBULATORY SURGICAL CENTERS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 80 EUROPE ACADEMIC RESEARCH INSTITUTES IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 81 EUROPE OTHERS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 82 EUROPE DERMAL FILLERS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 83 EUROPE DIRECT TENDER IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 84 EUROPE DRUG STORES IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 85 EUROPE RETAIL PHARMACY IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 86 EUROPE ONLINE PHARMACY IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 87 EUROPE OTHERS IN DERMAL FILLERS MARKET, BY REGION, 2020-2029 (USD MILLION)
图片列表
FIGURE 1 EUROPE DERMAL FILLERS MARKET: SEGMENTATION
FIGURE 2 EUROPE DERMAL FILLERS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE DERMAL FILLERS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE DERMAL FILLERS MARKET : EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE DERMAL FILLERS MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE DERMAL FILLERS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE DERMAL FILLERS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE DERMAL FILLERS MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 EUROPE DERMAL FILLERS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE DERMAL FILLERS MARKET: SEGMENTATION
FIGURE 11 RISING DEMAND FOR ANTI-AGING DRUGS, AND GROWING POPULARITY OF NON-SURGICAL OR MINIMALLY INVASIVE AESTHETICS PROCEDURES ARE EXPECTED TO DRIVE THE EUROPE DERMAL FILLERS MARKET IN THE FORECAST PERIOD 2022 TO 2029
FIGURE 12 BIODEGRADABLE DERMAL FILLER SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE DERMAL FILLERS MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE DERMAL FILLERS MARKET
FIGURE 14 EUROPE DERMAL FILLERS MARKET: BY PRODUCT TYPE, 2021
FIGURE 15 EUROPE DERMAL FILLERS MARKET: BY PRODUCT TYPE, 2022-2029 (USD MILLION)
FIGURE 16 EUROPE DERMAL FILLERS MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 17 EUROPE DERMAL FILLERS MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 EUROPE DERMAL FILLERS MARKET: BY MATERIAL TYPE, 2021
FIGURE 19 EUROPE DERMAL FILLERS MARKET, BY MATERIAL TYPE, 2022-2029 (USD MILLION)
FIGURE 20 EUROPE DERMAL FILLERS MARKET: BY MATERIAL TYPE, CAGR (2022-2029)
FIGURE 21 EUROPE DERMAL FILLERS MARKET: BY MATERIAL TYPE, LIFELINE CURVE
FIGURE 22 EUROPE DERMAL FILLERS MARKET : BY APPLICATION, 2021
FIGURE 23 EUROPE DERMAL FILLERS MARKET : BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 24 EUROPE DERMAL FILLERS MARKET : BY APPLICATION, CAGR (2022-2029)
FIGURE 25 EUROPE DERMAL FILLERS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 26 EUROPE DERMAL FILLERS MARKET: BY DRUG TYPE, 2021
FIGURE 27 EUROPE DERMAL FILLERS MARKET, BY DRUG TYPE, 2022-2029 (USD MILLION)
FIGURE 28 EUROPE DERMAL FILLERS MARKET: BY DRUG TYPE, CAGR (2022-2029)
FIGURE 29 EUROPE DERMAL FILLERS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 30 EUROPE DERMAL FILLERS MARKET: BY END USER, 2021
FIGURE 31 EUROPE DERMAL FILLERS MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 32 EUROPE DERMAL FILLERS MARKET: BY END USER, CAGR (2022-2029)
FIGURE 33 EUROPE DERMAL FILLERS MARKET: BY END USER, LIFELINE CURVE
FIGURE 34 EUROPE DERMAL FILLERS MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 35 EUROPE DERMAL FILLERS MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 36 EUROPE DERMAL FILLERS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 37 EUROPE DERMAL FILLERS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 38 EUROPE DERMAL FILLERS MARKET: SNAPSHOT (2021)
FIGURE 39 EUROPE DERMAL FILLERS MARKET: BY COUNTRY (2021)
FIGURE 40 EUROPE DERMAL FILLERS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 41 EUROPE DERMAL FILLERS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 42 EUROPE DERMAL FILLERS MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 43 EUROPE DERMA FILLERS MARKET: COMPANY SHARE 2021 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。













