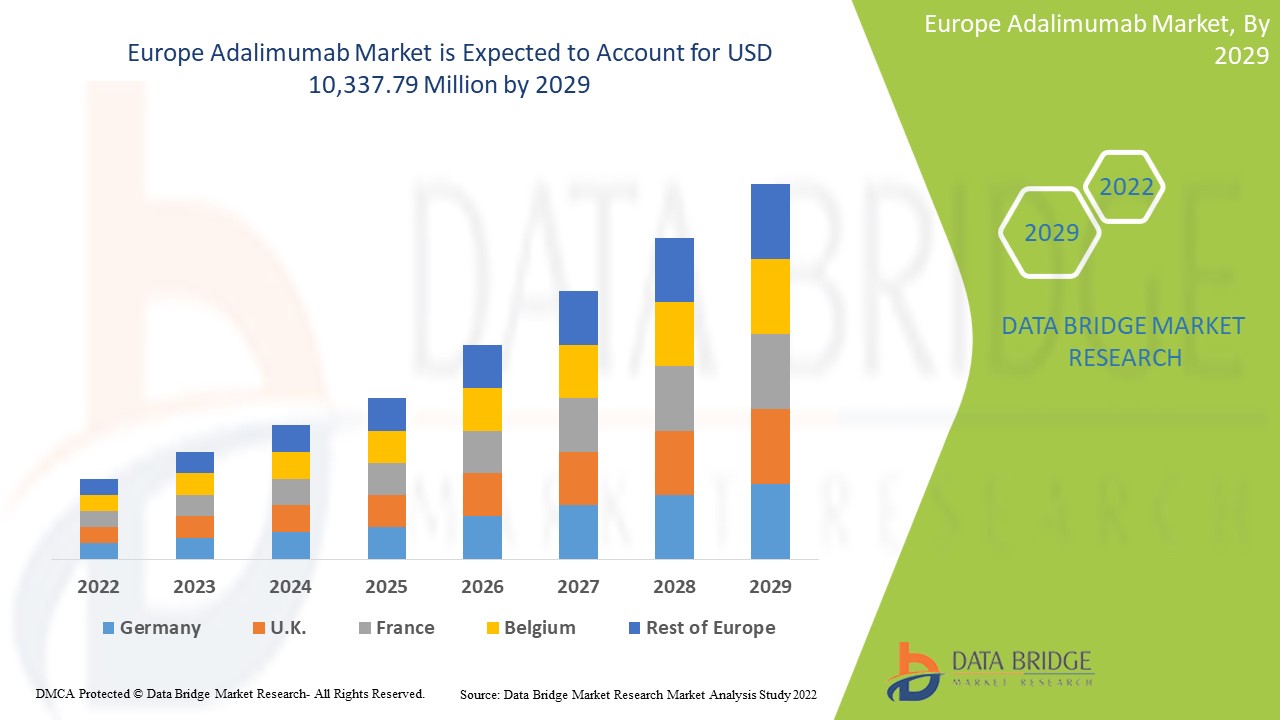
欧洲阿达木单抗市场,按适应症(类风湿性关节炎、幼年特发性关节炎、银屑病关节炎、强直性脊柱炎、克罗恩病、化脓性汗腺炎、溃疡性结肠炎、慢性斑块性银屑病、非感染性媒介等)、类型(生物制剂和生物仿制药)、剂量强度(20MG/0.4MLG、40MG/0.8MLG 等)、药物类型(Humira、Amgevita、Imraldi、Hyrimoz、Yuflyma、Hulio、Idacio)、人群类型(儿童和成人)、最终用户(医院、专科诊所、家庭医疗保健等)、分销渠道(医院药房、零售药房、网上药房等)行业趋势和预测到 2029 年。
市场定义
阿达木单抗是一种单克隆抗体,用于治疗某些自身免疫性疾病,包括类风湿性关节炎、克罗恩病等。阿达木单抗是一种抗 TNF 药物,用于治疗炎症症状。阿达木单抗的生物制剂是 Humira,也有各种 Humira 的生物仿制药,包括 Exemptia、Hyrimoz、Cyltezo 和 Hulio 等。阿达木单抗通过与 TNF 因子 alpha 结合起作用,从而降低自身免疫性疾病发生炎症反应的可能性。
市场分析和见解
预计欧洲阿达木单抗市场将在 2022 年至 2029 年的预测期内实现市场增长。Data Bridge Market Research 分析称,在 2022 年至 2029 年的预测期内,该市场将以 16.2% 的复合年增长率增长,预计到 2029 年将从 2021 年的 34.8594 亿美元达到 103.3779 亿美元。
|
报告指标 |
细节 |
|
预测期 |
2022 至 2029 年 |
|
基准年 |
2021 |
|
历史岁月 |
2020 |
|
定量单位 |
收入(百万美元) |
|
涵盖的领域 |
按适应症(类风湿性关节炎、幼年特发性关节炎、银屑病关节炎、强直性脊柱炎、克罗恩病、化脓性汗腺炎、溃疡性结肠炎、慢性斑块性银屑病、非感染性媒介及其他)、类型(生物制剂和生物仿制药)、剂量强度(20MG/0.4MLG、40MG/0.8MLG 及其他)、药物类型(Humira、Amgevita、Imraldi、Hyrimoz、Yuflyma、Hulio、Idacio)、人群类型(儿童和成人)、最终用户(医院、专科诊所、家庭医疗保健及其他)、分销渠道(医院药房、零售药房、网上药房及其他) |
|
覆盖国家 |
德国、法国、意大利、英国、西班牙、荷兰、俄罗斯、瑞士、比利时、土耳其、匈牙利、立陶宛、奥地利、爱尔兰、挪威、波兰和欧洲其他国家 |
|
涵盖的市场参与者 |
在市场上交易的主要公司包括 AbbVie Inc.、Sandoz International GmbH、Amgen Inc.、Mylan NV(Viatris 的子公司)、Biogen、Celltrion Healthcare Co., Ltd.、Fresenius Kabi SwissBioSim GmbH、Alvotech、Biocad、Coherus BioSciences、上海复宏汉霖生物科技股份有限公司、Synermore Biologics、Prestige BioPharma Ltd. 和 Janssen Global Services, LLC 等 |
阿达木单抗市场动态
驱动程序
- 类风湿关节炎患病率不断上升
类风湿性关节炎的发病率在世界范围内不断上升,据报道,全球每年每 10,000 人中就有近 3 例 RA。类风湿性关节炎会导致炎症症状的发展,可以通过多种药物疗法治疗,其中包括生物疗法。治疗类风湿性关节炎的最具创新性的生物疗法之一是阿达木单抗,这是一种针对免疫细胞的单克隆抗体,可减缓免疫细胞的募集,从而减少靶部位的炎症。全球和欧洲地区的类风湿性关节炎发病率持续上升,严重影响了患者的生活,因此疾病也给医疗保健专业人员带来了沉重的负担。
- 老年人口增加
老年人口越来越多,他们寿命越来越长,并且患有多种慢性疾病。据报道,人口老龄化速度过快,而且正在以惊人的速度增长。据说类风湿性关节炎与老年人口的增加有关。一项研究表明,2019 年约有 7.03 亿人年龄超过 65 岁或以上。北美和欧洲有超过 2 亿老年人。该研究还表明,人口已经明显老龄化,并以约 48% 的速度缓慢增长。患有类风湿性关节炎的成年人口正在增加,他们很大程度上依赖阿达木单抗治疗。这意味着老年人口的增加正在成为市场增长的驱动力。
- 合同研究组织 (CRO) 数量不断增加
合同研究组织为药物和医疗器械的临床试验和其他研究活动提供支持。合同研究组织 (CRO) 帮助生物技术和制药行业进行药物开发过程并降低临床试验的总体成本。由于各种阿达木单抗正在接受广泛的临床试验以满足患有炎症疾病的患者未满足的需求,因此合同研究组织的数量不断增加,推动了市场增长。合同研究组织的数量不断增加,简化了阿达木单抗药物的流程并缩短了药物开发所需的时间。
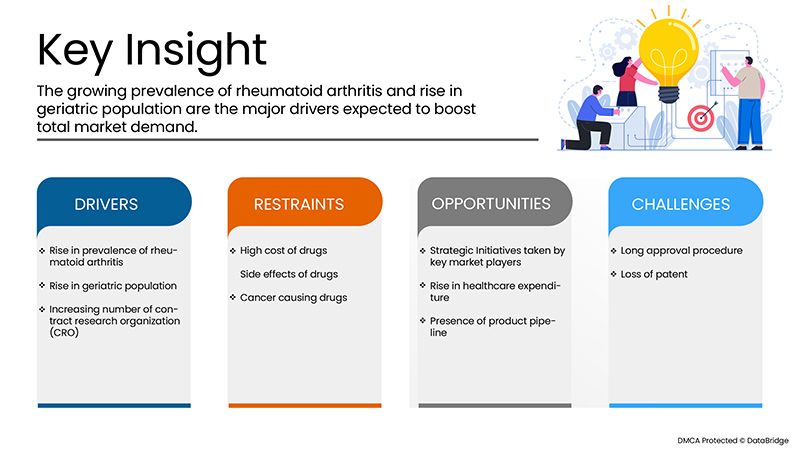
机会
- 市场参与者的战略举措
由于各种慢性疾病的发病率和老年人口的增加,该地区乃至全球对阿达木单抗的需求都有所增加。这些有利因素增加了对药物的需求,为了满足市场需求,大大小小的市场参与者都在采用各种策略
此外,该地区政府医疗保健支出的增加将为 2022-2029 年预测期内的阿达木单抗市场提供结构完整性和未来机会。
限制/挑战
然而,药物的高成本以及用药后出现的一些副作用可能会阻碍市场的增长。此外,在上述预测期内,严格的规则和法规将进一步挑战市场。
本阿达木单抗市场报告详细介绍了最新发展、贸易法规、进出口分析、生产分析、价值链优化、市场份额、国内和本地市场参与者的影响,分析了新兴收入领域的机会、市场法规的变化、战略市场增长分析、市场规模、类别市场增长、应用领域和主导地位、产品批准、产品发布、地域扩展、市场技术创新。如需了解有关关节炎市场的更多信息,请联系 Data Bridge Market Research 获取分析师简报,我们的团队将帮助您做出明智的市场决策,实现市场增长。
COVID-19 对阿达木单抗市场的影响
COVID-19 对市场增长的影响并不大。由于该地区各种疾病(如类风湿性关节炎、克罗恩病等)的患病率上升,导致市场上各种药物的开发增加。因此,在 COVID-19 期间,对阿达木单抗药物的需求也不断增加。
近期发展
- 2018年10月,诺华旗下子公司、生物仿制药领域的先驱和全球领导者山德士今天宣布,美国食品药品监督管理局 (FDA) 批准了其生物仿制药 HyrimozTM (阿达木单抗-adaz)。FDA 批准用于治疗类风湿性关节炎 (RA)、4 岁及以上患者的幼年特发性关节炎 (JIA)、银屑病关节炎 (PsA)、强直性脊柱炎 (AS)、成人克罗恩病 (CD)、溃疡性结肠炎 (UC) 和斑块状银屑病 (Ps)。
欧洲阿达木单抗市场范围
欧洲阿达木单抗市场细分为适应症、类型、剂量强度、药物类型、人群类型、最终用户和分销渠道。这些细分市场之间的增长将帮助您分析行业中增长微弱的细分市场,并为用户提供有价值的市场概览和市场洞察,以便做出战略决策,确定核心市场应用。
指征
- 类风湿关节炎
- 幼年特发性关节炎
- 银屑病关节炎
- 强直性脊柱炎
- 克罗恩病
- 化脓性汗腺炎
- 溃疡性结肠炎
- 慢性斑块状银屑病
- 非传染性中间体
- 其他的
根据适应症,欧洲阿达木单抗市场分为类风湿性关节炎、幼年特发性关节炎、银屑病关节炎、强直性脊柱炎、克罗恩病、化脓性汗腺炎、溃疡性结肠炎、慢性斑块状银屑病、非感染性中间体和其他。
类型
- 生物制剂
- 生物仿制药
根据类型,欧洲阿达木单抗市场分为生物制剂和生物仿制药。
剂量强度
- 20 毫克/0.4 毫升
- 40米/0.8米
- 其他的
根据剂量强度,欧洲阿达木单抗市场细分为 20MG/0.4MLG、40MG/0.8MLG 和其他。
药物类型
- 修美乐
- 安格维塔
- 伊姆拉尔迪
- 希里莫兹
- 尤夫利马
- 胡里奥
- 伊达西奥
根据药物类型,欧洲阿达木单抗市场分为 Humira、Amgevita、Imraldi、Hyrimoz、Yuflyma、Hulio 和 Idacio。
人口类型
- 孩子们
- 成年人
根据人口类型,欧洲阿达木单抗市场分为儿童和成人。
最终用户
- 医院
- 专科诊所
- 家庭医疗保健
- 其他的
根据最终用户,欧洲阿达木单抗市场分为医院、专科诊所、家庭医疗保健和其他。
分销渠道
- 医院药房
- 零售药店
- 网上药店
- 其他的
根据分销渠道,欧洲阿达木单抗市场分为医院药店、零售药店、网上药店和其他。
阿达木单抗市场区域分析/见解
对阿达木单抗市场进行了分析,并按国家、适应症、类型、剂量强度、药物类型、人群类型、最终用户和分销渠道提供了市场规模洞察和趋势。
欧洲阿达木单抗市场进一步细分为以下国家 - 德国、法国、意大利、英国、西班牙、荷兰、俄罗斯、瑞士、比利时、土耳其、匈牙利、立陶宛、奥地利、爱尔兰、挪威、波兰和欧洲其他地区。
德国在市场份额和市场收入方面占据阿达木单抗市场的主导地位,并将在预测期内继续保持主导地位。这是由于各种慢性病的患病率上升,而德国地区药物研发的研发进一步促进了这一市场的增长。
报告的国家部分还提供了影响单个市场因素和市场法规变化的信息,这些因素和变化会影响市场的当前和未来趋势。新旧销售、国家人口统计、疾病流行病学和进出口关税等数据点是预测单个国家市场情况的一些主要指标。此外,在对国家数据进行预测分析时,还考虑了全球品牌的存在和可用性以及它们因本土和国内品牌的激烈竞争而面临的挑战以及销售渠道的影响。
竞争格局和市场份额分析
阿达木单抗市场竞争格局提供了竞争对手的详细信息。详细信息包括公司概况、公司财务状况、产生的收入、市场潜力、研发投资、新市场计划、全球影响力、生产基地和设施、生产能力、公司优势和劣势、产品发布、产品宽度和广度以及应用主导地位。以上提供的数据点仅与公司对阿达木单抗市场的关注有关。
阿达木单抗市场的一些主要参与者包括 AbbVie Inc.、Sandoz International GmbH、Amgen Inc.、Mylan NV(Viatris 的子公司)、Biogen、Celltrion Healthcare Co., Ltd.、Fresenius Kabi SwissBioSim GmbH、Alvotech、Biocad、Coherus BioSciences、上海复宏汉霖生物科技股份有限公司、Synermore Biologics、Prestige BioPharma Ltd.、Janssen Global Services, LLC 等。
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析以及主要(行业专家)验证。除此之外,数据模型还包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、公司市场份额分析、测量标准、欧洲与地区和供应商份额分析。如有进一步询问,请要求分析师致电。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们很自豪能够为现有和新客户提供符合其目标的数据和分析。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(Factbook)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE ADALIMUMAB MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 INDICATION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PIPELINE ANALYSIS
4 REGULATORY FRAMEWORK OF EUROPE ADALIMUMAB MARKET
5 EPIDEMIOLOGY
6 ADALIMUMAB PRESCRIPTION
7 EUROPE ADALIMUMAB MARKET: REIMBURSEMENT SCENARIO
7.1 REIMBURSEMENT SCENARIO IN THE U.S.
7.2 REIMBURSEMENT SCENARIO IN CHINA
7.3 REIMBURSEMENT SCENARIO IN JAPAN
7.4 REIMBURSEMENT IN CENTRAL AND EASTERN EUROPE
7.5 REIMBURSEMENT SCENARIO IN DENMARK
7.6 REIMBURSEMENT SCENARIO IN IRELAND
8 IMPACT OF BIOSIMILAR
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN THE PREVALENCE OF RHEUMATOID ARHTRITIS
9.1.2 INCREASING GERIATRIC POPULATION
9.1.3 INCREASING NUMBER OF CONTRACT RESEARCH ORGANIZATIONS
9.1.4 INTRODUCTION TO BIOSIMILARS
9.1.5 EXPLORATION OF EMERGING MARKETS
9.2 RESTRAINTS
9.2.1 HIGH COSTS OF DRUGS
9.2.2 SIDE EFFECTS OF DRUGS
9.2.3 CANCER CAUSING DRUGS
9.3 OPPORTUNITIES
9.3.1 PRESENCE OF PRODUCT PIPELINE
9.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
9.3.3 INCREASING HEALTHCARE EXPENDITURE
9.3.4 PRESENCE OF REIMBURSEMENT POLICIES
9.4 CHALLENGES
9.4.1 LOSS OF PATENTS
9.4.2 AVAILABILITY OF ALTERNATIVES
9.4.3 LONG APPROVAL PROCEDURE
10 COVID-19 IMPACT ON ADALIMUMAB IN HEALTHCARE INDUSTRY
10.1 OVERVIEW
10.2 ADALIMUMAB AND COVID-19
10.3 PRICE IMPACT OF COVID-19
10.4 IMPACT ON DEMAND
10.5 IMPACT ON SUPPLY CHAIN
10.6 STRATEGIC DECISIONS FOR MANUFACTURERS
10.7 CONCLUSION
11 EUROPE ADALIMUMAB MARKET, BY INDICATION
11.1 OVERVIEW
11.2 RHEUMATOID ARTHRITIS
11.3 ANKYLOSING SPONDYLITIS
11.4 CHRONIC PLAQUE PSORIASIS
11.5 CROHN’S DISEASE
11.6 ULCERATIVE COLITIS
11.7 PSORIATIC ARTHRITIS
11.8 JUVENILE IDIOPATHIC ARTHRITIS
11.9 HIDRADENITIS SUPPURATIVA
11.1 NON-INFECTIOUS INTERMEDIATE
11.11 OTHERS
12 EUROPE ADALIMUMAB MARKET, BY TYPE
12.1 OVERVIEW
12.2 BIOLOGICS
12.3 BIOSIMILARS
12.3.1 ADALIMUMAB-ATTO
12.3.2 ADALIMUMAB-BWWD
12.3.3 ADALIMUMAB-ADBM
12.3.4 ADALIMUMAB-ADAZ
12.3.5 ADALIMUMAB-FKJP
12.3.6 ADALIMUMAB-AFZB
12.3.7 OTHERS
13 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGTH
13.1 OVERVIEW
13.2MG/0.4ML
13.3MG/0.8ML
13.4MG/0.4ML
13.5MG/0.1ML
13.6 OTHERS
14 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE
14.1 OVERVIEW
14.2 BRANDED
14.3 GENERICS
14.3.1 AMJEVITA
14.3.2 HYRIMOZ
14.3.3 HULIO
14.3.4 OTHERS
15 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 PARENTERAL
15.3 ORAL
16 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE
16.1 OVERVIEW
16.2 ADULTS
16.3 CHILDREN
17 EUROPE ADALIMUMAB MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 SPECIALTY CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACIES
18.3 RETAIL PHARMACIES
18.4 ONLINE PHARMACIES
18.5 DIRECT TENDER
18.6 OTHERS
19 EUROPE ADALIMUMAB MARKET, BY GEOGRAPHY
19.1 EUROPE
19.1.1 GERMANY
19.1.2 U.K
19.1.3 ITALY
19.1.4 FRANCE
19.1.5 SPAIN
19.1.6 NETHERLANDS
19.1.7 RUSSIA
19.1.8 SWITZERLAND
19.1.9 BELGIUM
19.1.10 TURKEY
19.1.11 AUSTRIA
19.1.12 NORWAY
19.1.13 HUNGARY
19.1.14 LITHUANIA
19.1.15 IRELAND
19.1.16 POLAND
19.1.17 REST OF EUROPE
20 EUROPE ADALIMUMAB MARKET: COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: EUROPE
21 SWOT
22 COMPANY PROFILES
22.1 ABBVIE INC.
22.1.1 COMPANY SNAPSHOT
22.1.2 REVENUE ANALYSIS
22.1.3 COMPANY SHARE ANALYSIS
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 AMGEN (EUROPE) GMBH (A SUBSIDIARY OF AMGEN INC.)
22.2.1 COMPANY SNAPSHOT
22.2.2 REVENUE ANALYSIS
22.2.3 COMPANY SHARE ANALYSIS
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 BIOGEN
22.3.1 COMPANY SNAPSHOT
22.3.2 REVENUE ANALYSIS
22.3.3 PRODUCT PORTFOLIO
22.3.4 RECENT DEVELOPMENTS
22.4 SANDOZ INTERNATIONAL GMBH {A SUBSIDIARY OF SANDOZ (A DIVISION OF NOVARTIS AG)}
22.4.1 COMPANY SNAPSHOT
22.4.2 REVENUE ANALYSIS
22.4.3 PRODUCT PORTFOLIO
22.4.4 RECENT DEVELOPMENTS
22.5 MYLAN N.V.
22.5.1 COMPANY SNAPSHOT
22.5.2 REVENUE ANALYSIS
22.5.3 PRODUCT PORTFOLIO
22.5.4 RECENT DEVELOPMENTS
22.6 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
22.6.1 COMPANY SNAPSHOT
22.6.2 REVENUE ANALYSIS
22.6.3 PRODUCT PORTFOLIO
22.6.4 RECENT DEVELOPMENTS
22.7 CELLTRION INC.
22.7.1 COMPANY SNAPSHOT
22.7.2 REVENUE ANALYSIS
22.7.3 PRODUCT PORTFOLIO
22.7.4 RECENT DEVELOPMENTS
22.8 COHERUS BIOSCIENCES
22.8.1 COMPANY SNAPSHOT
22.8.2 PRODUCT PORTFOLIO
22.8.3 RECENT DEVELOPMENTS
22.9 FRESENIUS KABI DEUTSCHLAND GMBH (A SUBSIDIARY OF FRESENIUS KABI AG)
22.9.1 COMPANY SNAPSHOT
22.9.2 REVENUE ANALYSIS
22.9.3 PRODUCT PORTFOLIO
22.9.4 RECENT DEVELOPMENTS
22.1 HETERO BIOPHARMA LTD.
22.10.1 COMPANY SNAPSHOT
22.10.2 PRODUCT PORTFOLIO
22.10.3 RECENT DEVELOPMENTS
22.11 INNOVENT BIOLOGICS, INC.
22.11.1 COMPANY SNAPSHOT
22.11.2 REVENUE ANALYSIS
22.11.3 PRODUCT PORTFOLIO
22.11.4 RECENT DEVELOPMENTS
22.12 PFIZER INC.
22.12.1 COMPANY SNAPSHOT
22.12.2 REVENUE ANALYSIS
22.12.3 PRODUCT PORTFOLIO
22.12.4 RECENT DEVELOPMENTS
22.13 RELIANCE LIFE SCIENCES (A SUBSIDIARY OF RELIANCE INDUSTRIES LIMITED)
22.13.1 COMPANY SNAPSHOT
22.13.2 REVENUE ANALYSIS
22.13.3 PRODUCT PORTFOLIO
22.13.4 RECENT DEVELOPMENTS
22.14 SAMSUNG BIOEPIS (A SUBSIDIARY OF SAMSUNG BIOLOGICS)
22.14.1 COMPANY SNAPSHOT
22.14.2 REVENUE ANALYSIS
22.14.3 PRODUCT PORTFOLIO
22.14.4 RECENT DEVELOPMENTS
22.15 ZYDUS CADILA
22.15.1 COMPANY SNAPSHOT
22.15.2 REVENUE ANALYSIS
22.15.3 PRODUCT PORTFOLIO
22.15.4 RECENT DEVELOPMENT
23 QUESTIONNAIRE
24 RELATED REPORTS
表格列表
LIST OF TABLES
TABLE 1 EUROPE ADALIMUMAB MARKET, PIPELINE ANALYSIS
TABLE 2 BIOSIMILAR OF ADALIMUMAB LAUNCHED IN THE U.S.
TABLE 3 PREVALENCE AND INCIDENCE RATES OF RA WORLDWIDE (CASE PER 100 INHABITANTS)
TABLE 4 BIOLOGIC DRUGS SUBJECTED TO PATENT LOSS
TABLE 5 ALTERNATIVE DRUGS FOR INFLAMMATORY DISEASES TREATMENT
TABLE 6 EUROPE ADALIMUMAB MARKET, BY INDICATION 2019-2027 (USD MILLION)
TABLE 7 EUROPE RHEUMATOID ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 8 EUROPE ANKYLOSING SPONDYLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 9 EUROPE CHRONIC PLAQUE PSORIASIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 10 EUROPE CROHN’S DISEASE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 11 EUROPE ULCERATIVE COLITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 12 EUROPE PSORIATIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 13 EUROPE JUVENILE IDIOPATHIC ARTHRITIS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 14 EUROPE HIDRADENITIS SUPPURATIVA IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 15 EUROPE NONINFECTIOUS INTERMEDIATE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 16 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 17 EUROPE ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 18 EUROPE BIOLOGICS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 19 EUROPE BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 20 EUROPE BIOSIMILARS IN ADALIMUMAB MARKET, BY TYPE 2019-2027 (USD MILLION)
TABLE 21 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGHT, 2019-2027 (USD MILLION)
TABLE 22 EUROPE 40MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 23 EUROPE 80MG/0.8ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 24 EUROPE 20MG/0.4ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 25 EUROPE 10MG/0.1ML IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 26 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 27 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE, 2019-2027 (USD MILLION)
TABLE 28 EUROPE BRANDED IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 29 EUROPE GENERICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 30 EUROPE GENERICS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 31 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION 2019-2027 (USD MILLION)
TABLE 32 EUROPE PARENTERAL IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 33 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE, 2019-2027 (USD MILLION)
TABLE 34 EUROPE ADULTS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 35 EUROPE CHILDREN IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 36 EUROPE ADALIMUMAB MARKET, BY END USER, 2019-2027 (USD MILLION)
TABLE 37 EUROPE HOSPITALS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 38 EUROPE SPECIALTY CLINICS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 39 EUROPE HOME HEALTHCARE IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 40 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 41 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
TABLE 42 EUROPE HOSPITAL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 43 EUROPE RETAIL PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 44 EUROPE ONLINE PHARMACIES IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 45 EUROPE DIRECT TENDER IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 46 EUROPE OTHERS IN ADALIMUMAB MARKET, BY REGION, 2017-2027 (USD MILLION)
TABLE 47 EUROPE ADALIMUMAB MARKET, BY COUNTRY, 2018-2027 (USD MILLION)
TABLE 48 EUROPE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 49 EUROPE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 50 EUROPE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 51 EUROPE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 52 EUROPE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 53 EUROPE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 54 EUROPE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 55 EUROPE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 56 EUROPE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 57 EUROPE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 58 GERMANYADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 59 GERMANYADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 60 GERMANYBIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 61 GERMANYADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 62 GERMANYADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 63 GERMANY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 64 GERMANYADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 65 GERMANYADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 66 GERMANYADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 67 GERMANYADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 68 U.K ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 69 U.K ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 70 U.K BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 71 U.K ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 72 U.K ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 73 U.K GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 74 U.K ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 75 U.K ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 76 U.K ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 77 U.K ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 78 ITALY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 79 ITALY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 80 ITALY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 81 ITALY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 82 ITALY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 83 ITALY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 84 ITALY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 85 ITALY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 86 ITALY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 87 ITALY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 88 FRANCE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 89 FRANCE ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 90 FRANCE BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 91 FRANCE ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 92 FRANCE ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 93 FRANCE GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 94 FRANCE ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 95 FRANCE ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 96 FRANCE ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 97 FRANCE ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 98 SPAIN ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 99 SPAIN ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 100 SPAIN BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 101 SPAIN ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 102 SPAIN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 103 SPAIN GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 104 SPAIN ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 105 SPAIN ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 106 SPAIN ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 107 SPAIN ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 108 NETHERLANDS ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 109 NETHERLANDS ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 110 NETHERLANDS BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 111 NETHERLANDS ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 112 NETHERLANDS ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 113 NETHERLANDS GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 114 NETHERLANDS ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 115 NETHERLANDS ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 116 NETHERLANDS ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 117 NETHERLANDS ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 118 RUSSIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 119 RUSSIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 120 RUSSIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 121 RUSSIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 122 RUSSIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 123 RUSSIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 124 RUSSIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 125 RUSSIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 126 RUSSIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 127 RUSSIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 128 SWITZERLAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 129 SWITZERLAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 130 SWITZERLAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 131 SWITZERLAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 132 SWITZERLAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 133 SWITZERLAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 134 SWITZERLAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 135 SWITZERLAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 136 SWITZERLAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 137 SWITZERLAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 138 BELGIUM ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 139 BELGIUM ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 140 BELGIUM BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 141 BELGIUM ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 142 BELGIUM ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 143 BELGIUM GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 144 BELGIUM ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 145 BELGIUM ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 146 BELGIUM ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 147 BELGIUM ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 148 TURKEY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 149 TURKEY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 150 TURKEY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 151 TURKEY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 152 TURKEY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 153 TURKEY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 154 TURKEY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 155 TURKEY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 156 TURKEY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 157 TURKEY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 158 AUSTRIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 159 AUSTRIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 160 AUSTRIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 161 AUSTRIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 162 AUSTRIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 163 AUSTRIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 164 AUSTRIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 165 AUSTRIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 166 AUSTRIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 167 AUSTRIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 168 NORWAY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 169 NORWAY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 170 NORWAY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 171 NORWAY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 172 NORWAY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 173 NORWAY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 174 NORWAY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 175 NORWAY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 176 NORWAY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 177 NORWAY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 178 HUNGARY ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 179 HUNGARY ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 180 HUNGARY BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 181 HUNGARY ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 182 HUNGARY ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 183 HUNGARY GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 184 HUNGARY ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 185 HUNGARY ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 186 HUNGARY ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 187 HUNGARY ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 188 LITHUANIA ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 189 LITHUANIA ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 190 LITHUANIA BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 191 LITHUANIA ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 192 LITHUANIA ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 193 LITHUANIA GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 194 LITHUANIA ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 195 LITHUANIA ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 196 LITHUANIA ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 197 LITHUANIA ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 198 IRELAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 199 IRELAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 200 IRELAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 201 IRELAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 202 IRELAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 203 IRELAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 204 IRELAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 205 IRELAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 206 IRELAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 207 IRELAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 208 POLAND ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
TABLE 209 POLAND ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 210 POLAND BIOSIMILARS OF ADALIMUMAB MARKET, BY TYPE, 2018-2027 (USD MILLION)
TABLE 211 POLAND ADALIMUMAB MARKET, BY DOSAGE STRENGTH, 2018-2027 (USD MILLION)
TABLE 212 POLAND ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 213 POLAND GENERICS IN ADALIMUMAB MARKET, BY DRUG TYPE, 2018-2027 (USD MILLION)
TABLE 214 POLAND ADALIMUMAB MARKET, BY ROUTE OF ADMINISTRATION, 2018-2027 (USD MILLION)
TABLE 215 POLAND ADALIMUMAB MARKET, BY POPULATION TYPE, 2018-2027 (USD MILLION)
TABLE 216 POLAND ADALIMUMAB MARKET, BY END USER, 2018-2027 (USD MILLION)
TABLE 217 POLAND ADALIMUMAB MARKET, BY DISTRIBUTION CHANNEL, 2018-2027 (USD MILLION)
TABLE 218 REST OF EUROPE ADALIMUMAB MARKET, BY INDICATION, 2018-2027 (USD MILLION)
图片列表
LIST OF FIGURES
FIGURE 1 EUROPE ADALIMUMAB MARKET: SEGMENTATION
FIGURE 2 EUROPE ADALIMUMAB MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE ADALIMUMAB MARKET: DROC ANALYSIS
FIGURE 4 EUROPE ADALIMUMAB MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE ADALIMUMAB MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE ADALIMUMAB MARKET: MULTIVARIATE MODELLING
FIGURE 7 EUROPE ADALIMUMAB MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE ADALIMUMAB MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE ADALIMUMAB MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE ADALIMUMAB MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF RHEUMATOID ARTHRITIS AND INCREASING GERIATRIC POPULATION IS DRIVING THE EUROPE ADALIMUMAB MARKET IN THE FORECAST PERIOD OF 2020 TO 2027
FIGURE 12 RHEUMATOID ARTHRITIS IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ADALIMUMAB MARKET IN 2020 & 2027
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE ADALIMUMAB MARKET
FIGURE 14 MARKET GROWTH IN CLINICAL CRO (IN USD MILLIONS)
FIGURE 15 FUNCTION OF CRO
FIGURE 16 HEALTHCARE EXPENDITURE IN 2016 AND 2019
FIGURE 17 EUROPE ADALIMUMAB MARKET: BY INDICATION, 2019
FIGURE 18 EUROPE ADALIMUMAB MARKET: BY INDICATION, 2019-2027 (USD MILLION)
FIGURE 19 EUROPE ADALIMUMAB MARKET: BY INDICATION, CAGR (2020-2027)
FIGURE 20 EUROPE ADALIMUMAB MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 21 EUROPE ADALIMUMAB MARKET: BY TYPE, 2019
FIGURE 22 EUROPE ADALIMUMAB MARKET: BY TYPE 2019-2027 (USD MILLION)
FIGURE 23 EUROPE ADALIMUMAB MARKET: BY TYPE, CAGR (2020-2027)
FIGURE 24 EUROPE ADALIMUMAB MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, 2019
FIGURE 26 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH 2019-2027 (USD MILLION)
FIGURE 27 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, CAGR (2020-2027)
FIGURE 28 EUROPE ADALIMUMAB MARKET: BY DOSAGE STRENGTH, LIFELINE CURVE
FIGURE 29 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, 2019
FIGURE 30 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE , 2019-2027 (USD MILLION)
FIGURE 31 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, CAGR (2020-2027)
FIGURE 32 EUROPE ADALIMUMAB MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 33 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019
FIGURE 34 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, 2019-2027 (USD MILLION)
FIGURE 35 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2020-2027)
FIGURE 36 EUROPE ADALIMUMAB MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 37 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, 2019
FIGURE 38 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, 2019-2027 (USD MILLION)
FIGURE 39 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, CAGR (2020-2027)
FIGURE 40 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE, LIFELINE CURVE
FIGURE 41 EUROPE ADALIMUMAB MARKET: BY END USER, 2019
FIGURE 42 EUROPE ADALIMUMAB MARKET: BY END USER, 2019-2027 (USD MILLION)
FIGURE 43 EUROPE ADALIMUMAB MARKET: BY END USER, CAGR (2020-2027)
FIGURE 44 EUROPE ADALIMUMAB MARKET: BY END USER, LIFELINE CURVE
FIGURE 45 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019
FIGURE 46 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, 2019-2027 (USD MILLION)
FIGURE 47 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, CAGR (2020-2027)
FIGURE 48 EUROPE ADALIMUMAB MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 49 EUROPE ADALIMUMAB MARKET: SNAPSHOT (2019)
FIGURE 50 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2019)
FIGURE 51 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2020 & 2027)
FIGURE 52 EUROPE ADALIMUMAB MARKET: BY COUNTRY (2019 & 2027)
FIGURE 53 EUROPE ADALIMUMAB MARKET: BY POPULATION TYPE (2020-2027)
FIGURE 54 EUROPE ADALIMUMAB MARKET: COMPANY SHARE 2019 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。