亚太地区 CRISPR 基因检测和诊断市场,按类别(第 1 类 - 多种效应蛋白和第 2 类 - 单个 CrRNA 结合蛋白)、产品和服务(产品和服务)、应用(生物医学诊断、基因组工程、药物发现、农业应用等)、工作流程(样品制备、预扩增、CrRNA、Cas 酶和传感)、最终用户(医院、诊断中心、生物技术公司、学术和研究机构等)、分销渠道(直接招标、零售销售)划分的行业趋势和预测到 2029 年
市场定义和见解
CRISPR 是一种基因组编辑工具,它允许研究人员轻松改变 DNA 序列和修改基因功能。它有许多潜在的应用,包括纠正遗传缺陷以及治疗和预防疾病传播。基于 CRISPR 的诊断方法已用于许多生物医学应用,例如检测传染性和非传染性疾病的核酸生物标志物以及检测遗传疾病。CRISPR 中的检测试剂盒由两部分组成:一种称为 Cas9 的蛋白质和一种向导 RNA,即一串具有特定遗传密码的核酸分子。
该 CRISPR-Cas9 系统已针对哺乳动物细胞进行了修改。我们可以通过非同源末端连接 (NHEJ) 引入移码突变,从而引入特定于我们感兴趣的基因的引导序列 (sgRNA) 来敲除特定基因,或者产生敲入突变。
CRISPR-Cas 9 系统扩大了基因和细胞治疗的诊断和服务范围。制药公司在研发方面投入巨资以开发新产品,大量基因和细胞治疗药物进入早期开发阶段。市场参与者的投资将为急需治疗的患者提供安全有效的治疗方案。
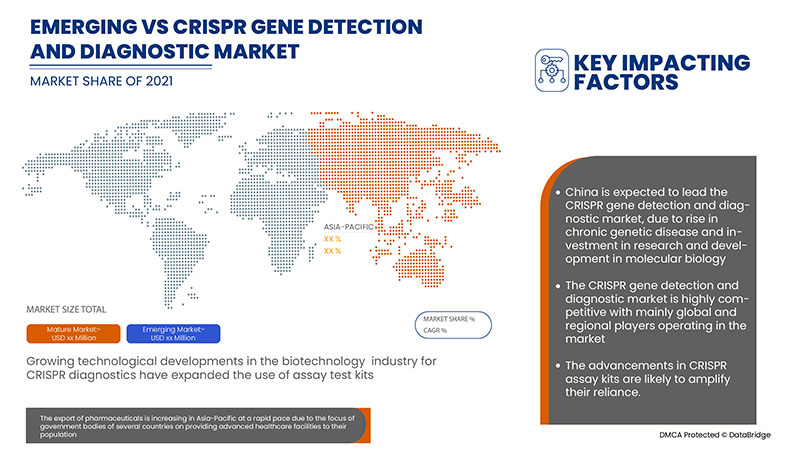
亚太地区 CRISPR 基因检测和诊断具有支持作用,旨在减轻症状的严重程度。Data Bridge Market Research 分析称,在 2022 年至 2029 年的预测期内,CRISPR 基因检测和诊断市场将以 21.6% 的复合年增长率增长。
|
报告指标 |
细节 |
|
预测期 |
2022 至 2029 年 |
|
基准年 |
2021 |
|
历史岁月 |
2020(可定制至 2019 - 2014) |
|
定量单位 |
收入(百万美元),定价(美元) |
|
涵盖的领域 |
按类别(第 1 类 - 多种效应蛋白和第 2 类 - 单个 CrRNA 结合蛋白)、产品和服务(产品和服务)、应用(生物医学诊断、基因组工程、药物发现、农业应用等)、工作流程(样品制备、预扩增、CrRNA、Cas 酶和传感)、最终用户(医院、诊断中心、生物技术公司、学术和研究机构等)分销渠道(直接招标、零售销售)、 |
|
覆盖国家 |
中国、日本、韩国、印度、澳大利亚、新加坡、泰国、马来西亚、印度尼西亚、菲律宾及亚太地区其他地区 |
|
涵盖的市场参与者 |
GenScript、Takara Bio Inc.、OriGene Technologies, Inc.、Agilent Technologies, Inc.、Synthego、Merck KGaA、Integrated DNA Technologies, Inc.(丹纳赫的子公司)和Thermo Fisher Scientific Inc. 等。 |
亚太地区 CRISPR 基因检测和诊断市场动态
驱动程序
- 慢性病患病率和发病率上升
慢性病是常见的疾病,每3个成年人中就有1人患有慢性病,慢性病影响着广大人民群众的健康和生活质量。
CRISPR 是成簇的规律间隔的短回文重复序列的缩写。近年来,CRISPR 已成为基因编辑的主导工具,用于改变细胞中 DNA 的特定序列。CRISPR 在亨廷顿氏病、肌肉营养不良症、癌症和高胆固醇的研究和治疗中具有重要用途。
例如,
- 2021 年,NORD(美国罕见疾病组织)的数据显示,杜氏肌营养不良症 (DMD) 的诊断发病率很高。杜氏肌营养不良症 (DMD) 是一种常见的遗传病,全球每 3,500 名男性新生儿中就有 1 名患有此病
- 研发投资增加
基因编辑技术,例如 CRISPR-Cas 9 系统,已经扩大了基因和细胞治疗的诊断和服务范围。制药公司在研发方面投入大量资金来开发新产品,大量基因和细胞治疗药物进入早期开发阶段。市场参与者的投资将实现为急需的患者提供安全有效的治疗的目标。
例如,
- 2022 年 2 月,Synthego 筹集了 2 亿美元用于研发,以推动基于 CRISPR 的药物从早期研究到临床的开发。Synthego 将利用 E 轮融资的投资金额来加速创建 CRISPR 诊断和服务
CRISPR 基因诊断的资金可用性
CRISPR 基因诊断和研究由美国国立卫生研究院 (NIH) 预算资助。私营部门也资助 CRISPR 基因检测和研究,但这种投资通常发生在测试和开发阶段,然后是初始基础研究。由于基因组编辑是一个如此新的领域,必须有一个公正的政府机构对其进行监督;FDA 谨慎而彻底,但他们在无休止地争取资金,进行长期投资,使付款与潜在的未来受益者保持一致,这将进一步促进 CRISPR 基因检测和诊断市场的增长。
此外,CRISPR 基因诊断技术的进步、公共和私人组织不断增加的宣传活动以及政府资金的增加都是扩大亚太地区 CRISPR 基因检测市场的因素。其他因素,如对有效疗法的需求增加以及对及时诊断的认识不断提高,将对 CRISPR 基因检测和诊断市场的增长率产生积极影响。此外,高可支配收入、慢性病患者数量的增加、生活方式的改变将导致 CRISPR 基因检测和诊断市场的扩大。
机会
- 医疗支出的增加
此外,研发活动的增加以及政府和私人组织投资的增加将为市场增长率带来新的机遇。
- 市场参与者的战略主动性
由于对慢性病的及时治疗,美国和亚太地区对 CRISPR 基因检测和诊断的需求增加。这些有利因素增加了对药物的需求,为了满足市场需求,大大小小的市场参与者正在采用各种策略。
主要参与者还试图制定具体的策略,例如产品发布、收购、批准、扩张和合作,以确保业务顺利进行,避免风险,并增加市场销售的长期增长。
例如,
- 2021 年 5 月,Horizon Discovery Ltd. 在沃尔瑟姆扩展了基因调节产品组合,推出了首个合成单向导 RNA 和正在申请专利的用于 CRISPR 干扰的 dcas9 抑制剂。产品组合的扩展增加了合成向导 RNA 产品组合在美国和英国地区的销售和收入,并增加了与市场参与者的合作
此外,有效疗法的推出和持续的临床试验将为 2022-2029 年预测期内的 CRISPR 基因检测和诊断市场提供有利机会。此外,当前未满足的大量需求和医疗技术的发展将在未来提高 CRISPR 基因检测和诊断市场的增长率。
限制/挑战
然而,CRISPR 诊断的高成本和使用 CRISPR 诊断时面临的风险将阻碍 CRISPR 基因检测和诊断市场的增长率。此外,使用 MRI 设备时产生的风险也将阻碍 CRISPR 基因检测和诊断市场的增长。缺乏熟练的专业知识和法规将在上述预测期内进一步挑战市场。
- 基于 CRISPR 的诊断成本上升
基于 CRISPR 的疗法具有巨大的潜力,但成本较高。大多数基因组编辑疗法需要更长的开发和生产时间,因此成本上升。此外,与 CRISPR 基因检测和诊断相关的检测试剂盒和药物适用于大部分人群。这些成本被转嫁给患者。因此,目前的高成本预计未来将呈下降趋势。
例如,
- 2021 年 7 月,据 Integrated DNA Technologies, Inc. 称,首个基于 CRISPR 的 SARS-CoV-2 商业化诊断检测试剂盒(包括逆转录 LAMP (RT-LAMP) 作为预扩增)目前售价为每次反应 30.15 美元
CRISPR 基因检测和诊断市场报告提供了最新发展、贸易法规、进出口分析、生产分析、价值链优化、市场份额、国内和本地市场参与者的影响的详细信息,分析了新兴收入领域的机会、市场法规的变化、战略市场增长分析、市场规模、类别市场增长、应用领域和主导地位、产品批准、产品发布、地域扩展、市场技术创新。如需了解有关 CRISPR 基因检测和诊断市场的更多信息,请联系 Data Bridge Market Research 获取分析师简报,我们的团队将帮助您做出明智的市场决策,实现市场增长。
患者流行病学分析
根据Globocan的研究,2020年乳腺癌的发病率较高,约为11.7%,其次是肺癌(11.40%)、结直肠癌(10.00%),宫颈癌和食道癌的发病率较低。
CRISPR 基因检测和诊断市场还为您提供有关患者分析、预后和治疗的详细市场分析。患病率、发病率、死亡率、依从率是报告中提供的一些数据变量。分析流行病学对市场增长的直接或间接影响,以创建更稳健、更队列的多元统计模型,用于预测增长期的市场。
COVID-19 对CRISPR 基因检测和诊断市场的影响
COVID-19 给市场带来了负面影响。疫情期间的封锁和隔离使诊断管理和治疗变得复杂。无法获得常规和药物管理的医疗设施将进一步影响市场。社会隔离会增加压力、绝望和社会支持,所有这些都可能导致疫情期间抗惊厥药物依从性的降低。
近期发展
- 2020 年 8 月,SHERLOCK BIOSCIENCES 宣布与 Dartmouth-Hitchcock Health 合作,开展 SHERLOCK 新冠病毒诊断试剂盒临床试验。该试剂盒已获得美国食品药品监督管理局 (FDA) 的紧急使用授权 (EUA) 紧急批准。
亚太地区 CRISPR 基因检测和诊断市场范围
CRISPR基因检测和诊断市场分为六个部分:类别、产品和服务、应用、工作流程、最终用户和分销渠道。这些部分之间的增长将帮助您分析行业中微弱的增长部分,并为用户提供有价值的市场概览和市场洞察,帮助他们做出战略决策,确定核心市场应用 。
班级
- 第 1 类 - 多重效应蛋白
- 第 2 类 - 单个 CrRNA 结合蛋白
根据类别,亚太地区 CRISPR 基因检测和诊断市场细分为第 1 类 - 多种效应蛋白和第 2 类 - 单个 CrRNA 结合蛋白。
产品与服务
- 产品
- 服务
根据产品和服务,亚太地区 CRISPR 基因检测和诊断市场细分为产品和服务。
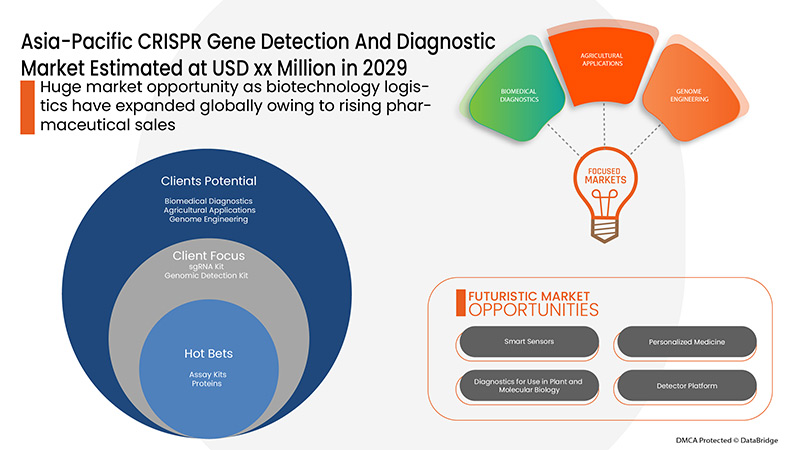
应用
- 生物医学诊断
- 基因组工程
- 药物研发
- 农业应用
- 其他的
根据应用,亚太地区 CRISPR 基因检测和诊断市场细分为生物医学诊断、基因组工程、药物发现、农业应用和其他。
工作流
- 样品制备
- 前置放大
- 肌动蛋白RNA
- Cas酶
- 传感
根据工作流程,亚太地区 CRISPR 基因检测和诊断市场细分为样品制备、预扩增、CrRNA、Cas 酶和传感。
最终用户
- 医院
- 诊断中心
- 生物科技公司
- 学术及研究机构
- 其他的
根据最终用户,亚太地区 CRISPR 基因检测和诊断市场分为医院、诊断中心、生物技术公司、学术和研究机构等。
分销渠道
- 直接招标
- 零售销售
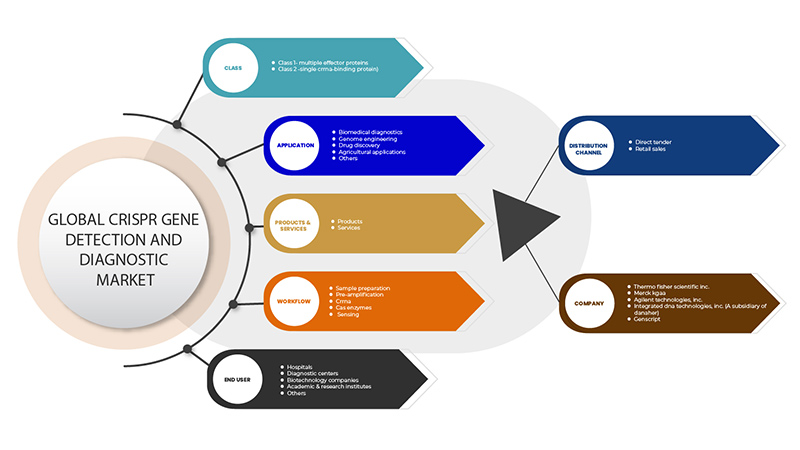
根据分销渠道,亚太地区 CRISPR 基因检测和诊断市场分为直接招标和零售。
CRISPR 基因检测和诊断市场区域分析/见解
The Asia-Pacific CRISPR gene detection and diagnostic market is analysed and market size insights and trends are provided by regions, class, products & services, application, workflow, end user, and distribution channel as referenced above.
The countries covered in the CRISPR gene detection and diagnostic market report are China, Japan, South Korea, India, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines And Rest of Asia-Pacific.
The China dominates the CRISPR gene detection and diagnostic market due to the rise in clinical trials for CRISPR based diagnostics.
The country section of the report also provides individual market impacting factors and changes in regulations in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of Asia-Pacific brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and CRISPR Gene Detection and Diagnostic Market Share Analysis
The Asia-Pacific CRISPR gene detection and diagnostic market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, the Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the CRISPR gene detection and diagnostic market.
Some of the major players operating in the CRISPR gene detection and diagnostic market are GenScript, Takara Bio Inc., OriGene Technologies, Inc., Agilent Technologies, Inc., Synthego, Merck KGaA, Integrated DNA Technologies, Inc. (A subsidiary of Danaher) Thermo Fisher Scientific Inc. among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
目录
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 CLASS SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 INTELLECTUAL PROPERTY LANDSCAPE (PATENT LANDSCAPE)
6 EPIDEMIOLOGY
7 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: REGULATORY SCENARIO
8 PIPELINE ANALYSIS FOR ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, OF CRISPR DIAGNOSTICS
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN PREVALENCE AND INCIDENCE OF CHRONIC DISEASES
9.1.2 RISE IN INVESTMENT IN RESEARCH AND DEVELOPMENT
9.1.3 AVAILABILITY OF FUNDING FOR CRISPR GENE DIAGNOSTICS
9.1.4 RISE IN GMP-CERTIFICATION APPROVALS FOR CRISPR GENE DIAGNOSTIC
9.1.5 RISE IN CLINICAL TRIALS FOR CRISPR BASED DIAGNOSTICS
9.2 RESTRAINTS
9.2.1 RISE IN COST OF CRISPR BASED DIAGNOSTICS
9.2.2 RISKS FACED WHILE USING CRISPR DIAGNOSIS
9.2.3 ETHICAL CONCERNS RELATED TO CRISPR GENE DETECTION AND DIAGNOSTIC RESEARCH
9.2.4 AVAILABILITY OF ALTERNATIVES
9.3 OPPORTUNITIES
9.3.1 STRATEGIC INITIATIVE BY MARKET PLAYERS
9.3.2 RISE IN HEALTHCARE EXPENDITURE
9.3.3 EMERGENCE OF TECHNOLOGICAL ADVANCEMENTS IN CRISPR BASED DIAGNOSTICS
9.4 CHALLENGES
9.4.1 LACK OF SKILLED PROFESSIONALS REQUIRED FOR CRISPR DIAGNOSTICS
9.4.2 STRINGENT REGULATIONS
10 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS
10.1 OVERVIEW
10.2 CLASS-2 SINGLE CRRNA-BINDING PROTEIN
10.2.1 BIOMEDICAL DIAGNOSTICS
10.2.2 AGRICULTURAL APPLICATIONS
10.2.3 GENOME ENGINEERING
10.2.4 DRUG DISCOVERY
10.2.5 OTHERS
10.3 CLASS-1 MULTIPLE EFFECTOR PROTEINS
11 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES
11.1 OVERVIEW
11.2 PRODUCTS
11.2.1 ASSAY KITS
11.2.1.1 SGRNA KIT
11.2.1.2 GENOMIC DETECTION KIT
11.2.1.3 OTHERS
11.2.2 PROTEINS
11.2.2.1 CAS9
11.2.2.2 CPF1
11.2.2.3 OTHERS
11.2.3 PLASMID AND VECTOR
11.2.4 LIBRARY
11.2.5 CONTROL KITS
11.2.6 DELIVERY SYSTEM PRODUCTS
11.2.7 DESIGN TOOLS
11.2.8 GENOMIC RNA
11.2.9 HDR BLOCKERS
11.2.9.1 AZIDOTHYMIDINE
11.2.9.2 TRIFLUOROTHYMIDINE
11.2.9.3 OTHERS
11.2.9.4 OTHERS
11.3 SERVICES
11.3.1 G-RNA DESIGN
11.3.2 CELL LINE ENGINEERING
11.3.3 MICROBIAL GENE EDITING
11.3.4 DNA SYNTHESIS
11.3.5 OTHERS
12 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION
12.1 OVERVIEW
12.2 BIOMEDICAL DIAGNOSTICS
12.2.1 CANCER
12.2.2 BLOOD DISORDERS
12.2.3 HEREDITARY DISORDERS
12.2.4 MUSCULAR DYSTROPHY
12.2.5 AIDS
12.2.6 NEURODEGENERATIVE CONDITION
12.2.7 OTHERS
12.3 AGRICULTURAL APPLICATIONS
12.4 GENOME ENGINEERING
12.4.1 CELL LINE ENGINEERING
12.4.2 HUMAN STEM CELLS
12.5 DRUG DISCOVERY
12.6 OTHERS
13 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW
13.1 OVERVIEW
13.2 CRRNA
13.3 CAS ENZYME
13.4 PRE-AMPLIFICATION
13.4.1 PCR
13.4.2 LAMP
13.4.3 RPA
13.5 SAMPLE PREPARATION
13.6 SENSING
13.6.1 FLUORESCENT PROBES
13.6.2 COLORIMETRIC
14 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER
14.1 OVERVIEW
14.2 BIOTECHNOLOGY COMPANIES
14.3 ACADEMIC AND RESEARCH INSTITUTES
14.4 DIAGNOSTIC CENTERS
14.5 HOSPITALS
14.6 OTHERS
15 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
16 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION
16.1 ASIA-PACIFIC
16.1.1 CHINA
16.1.2 JAPAN
16.1.3 INDIA
16.1.4 SOUTH KOREA
16.1.5 AUSTRALIA
16.1.6 SINGAPORE
16.1.7 THAILAND
16.1.8 PHILIPPINES
16.1.9 MALAYSIA
16.1.10 INDONESIA
16.1.11 REST OF ASIA-PACIFIC
17 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 THERMO FISHER SCIENTIFIC INC.
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENTS
19.2 MERCK KGA
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 AGILENT TECHNILOGIES, INC
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENT
19.4 INTEGRATED DNA TECHNOLOGIES, INC. (A SUBSIDIARY OF DANAHER)
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENTS
19.5 GENSCRIPT
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.6 10 X GENOMICS
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.7 APPLIED STEM CELL
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 ADDGENE
19.8.1 COMPANY SNAPSHOT
19.8.2 PRODUCT PORTFOLIO
19.8.3 RECENT DEVELOPMENT
19.9 BIOVISION INC.
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENT
19.1 CELLECTA, INC
19.10.1 COMPANY SNAPSHOT
19.10.2 PRODUCT PORTFOLIO
19.10.3 RECENT DEVELOPMENTS
19.11 CAS TAG BIOSCIENCES
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 GENECOPOEIA, INC.
19.12.1 COMPANY SNAPSHOT
19.12.2 PRODUCT PORTFOLIO
19.12.3 RECENT DEVELOPMENT
19.13 HORIZON DISCOVERY LTD
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENTS
19.14 HERA BIOLABS
19.14.1 COMPANY SNAPSHOT
19.14.2 PRODUCT PORTFOLIO
19.14.3 RECENT DEVELOPMENT
19.15 NEW ENGLAND BIOLABS
19.15.1 COMPANY SNAPSHOT
19.15.2 PRODUCT PORTFOLIO
19.15.3 RECENT DEVELOPMENTS
19.16 ORIGENE TECHNOLOGIES, INC.
19.16.1 COMPANY SNAPSHOT
19.16.2 PRODUCT PORTFOLIO
19.16.3 RECENT DEVELOPMENT
19.17 SYNTHEGO
19.17.1 COMPANY SNAPSHOT
19.17.2 PRODUCT PORTFOLIO
19.17.3 RECENT DEVELOPMENTS
19.18 TAKARA BIO INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
19.19 TOOLGEN, INC.
19.19.1 COMPANY SNAPSHOT
19.19.2 PRODUCT PORTFOLIO
19.19.3 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
表格列表
TABLE 1 PIPELINE ANALYSIS FOR ASIA PACIFIC CRISPR GENE THERAPEUTICS
TABLE 2 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 3 ASIA PACIFIC CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 ASIA PACIFIC CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 5 ASIA PACIFIC CLASS-1 MULTIPLE EFFECTOR PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 7 ASIA PACIFIC PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 ASIA PACIFIC PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 9 ASIA PACIFIC ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 10 ASIA PACIFIC PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 11 ASIA PACIFIC HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 12 ASIA PACIFIC SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 ASIA PACIFIC SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 14 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 15 ASIA PACIFIC BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 ASIA PACIFIC BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 17 ASIA PACIFIC AGRICULTURAL APPLICATIONS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 ASIA PACIFIC GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 ASIA PACIFIC GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 20 ASIA PACIFIC DRUG DISCOVERYIN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 ASIA PACIFIC OTHERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 23 ASIA PACIFIC CRRNA IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 ASIA PACIFIC CAS ENZYME IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 ASIA PACIFIC PRE-AMPLIFICATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 ASIA PACIFIC PRE-AMPLIFICATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 27 ASIA PACIFIC SAMPLE PREPARATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 ASIA PACIFIC SENSING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 ASIA PACIFIC SENSING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 30 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 31 ASIA PACIFIC BIOTECHNOLOGY COMPANIES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 ASIA PACIFIC ACADEMIC AND RESEARCH INSTITUTES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 ASIA PACIFIC DIAGNOSTIC CENTERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 ASIA PACIFIC HOSPITALS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 ASIA PACIFIC OTHERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 37 ASIA PACIFIC DIRECT TENDER IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 ASIA PACIFIC RETAIL SALES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 40 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 41 ASIA-PACIFIC CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 42 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 43 ASIA-PACIFIC PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 44 ASIA-PACIFIC ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 45 ASIA-PACIFIC HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 46 ASIA-PACIFIC PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 47 ASIA-PACIFIC SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 48 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 49 ASIA-PACIFIC GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 50 ASIA-PACIFIC BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 51 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 52 ASIA-PACIFIC PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 53 ASIA-PACIFIC SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 54 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 CHINA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 57 CHINA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 58 CHINA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 59 CHINA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 60 CHINA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 61 CHINA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 62 CHINA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 63 CHINA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 CHINA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 65 CHINA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 66 CHINA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 CHINA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 68 CHINA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 69 CHINA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 70 CHINA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 CHINA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 72 JAPAN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 73 JAPAN CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 74 JAPAN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 75 JAPAN PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 JAPAN ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 77 JAPAN HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 78 JAPAN PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 79 JAPAN SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 80 JAPAN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 81 JAPAN GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 82 JAPAN BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 83 JAPAN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 84 JAPAN PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 85 JAPAN SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 86 JAPAN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 87 JAPAN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 88 INDIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 89 INDIA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 90 INDIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 91 INDIA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 92 INDIA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 93 INDIA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 94 INDIA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 95 INDIA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 96 INDIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 97 INDIA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 98 INDIA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 99 INDIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 100 INDIA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 101 INDIA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 102 INDIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 103 INDIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 104 SOUTH KOREA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 105 SOUTH KOREA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 106 SOUTH KOREA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 107 SOUTH KOREA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 108 SOUTH KOREA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 109 SOUTH KOREA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 110 SOUTH KOREA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 111 SOUTH KOREA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 112 SOUTH KOREA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 113 SOUTH KOREA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 114 SOUTH KOREA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 115 SOUTH KOREA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 116 SOUTH KOREA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 117 SOUTH KOREA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 118 SOUTH KOREA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 119 SOUTH KOREA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 120 AUSTRALIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 121 AUSTRALIA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 122 AUSTRALIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 123 AUSTRALIA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 124 AUSTRALIA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 125 AUSTRALIA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 126 AUSTRALIA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 127 AUSTRALIA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 128 AUSTRALIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 129 AUSTRALIA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 130 AUSTRALIA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 131 AUSTRALIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 132 AUSTRALIA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 133 AUSTRALIA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 134 AUSTRALIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 135 AUSTRALIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 136 SINGAPORE CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 137 SINGAPORE CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 138 SINGAPORE CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 139 SINGAPORE PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 140 SINGAPORE ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 141 SINGAPORE HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 142 SINGAPORE PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 143 SINGAPORE SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 144 SINGAPORE CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 145 SINGAPORE GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 146 SINGAPORE BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 147 SINGAPORE CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 148 SINGAPORE PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 149 SINGAPORE SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 150 SINGAPORE CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 151 SINGAPORE CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 152 THAILAND CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 153 THAILAND CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 154 THAILAND CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 155 THAILAND PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 156 THAILAND ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 157 THAILAND HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 158 THAILAND PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 159 THAILAND SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 160 THAILAND CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 161 THAILAND GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 162 THAILAND BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 163 THAILAND CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 164 THAILAND PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 165 THAILAND SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 166 THAILAND CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 167 THAILAND CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 168 PHILIPPINES CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 169 PHILIPPINES CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 170 PHILIPPINES CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 171 PHILIPPINES PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 172 PHILIPPINES ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 173 PHILIPPINES HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 174 PHILIPPINES PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 175 PHILIPPINES SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 176 PHILIPPINES CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 177 PHILIPPINES GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 178 PHILIPPINES BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 179 PHILIPPINES CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 180 PHILIPPINES PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 181 PHILIPPINES SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 182 PHILIPPINES CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 183 PHILIPPINES CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 184 MALAYSIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 185 MALAYSIA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 186 MALAYSIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 187 MALAYSIA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 188 MALAYSIA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 189 MALAYSIA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 190 MALAYSIA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 191 MALAYSIA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 192 MALAYSIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 193 MALAYSIA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 194 MALAYSIA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 195 MALAYSIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 196 MALAYSIA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 197 MALAYSIA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 198 MALAYSIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 199 MALAYSIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 200 INDONESIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 201 INDONESIA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 202 INDONESIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 203 INDONESIA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 204 INDONESIA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 205 INDONESIA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 206 INDONESIA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 207 INDONESIA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 208 INDONESIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 209 INDONESIA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 210 INDONESIA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 211 INDONESIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 212 INDONESIA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 213 INDONESIA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 214 INDONESIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 215 INDONESIA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 216 REST OF ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
图片列表
FIGURE 1 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DBMR POSITION GRID
FIGURE 8 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: END USER COVERAGE GRID
FIGURE 10 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED INCIDENCE OF CHRONIC DISEASES, RISE IN TECHNOLOGICAL ADVANCEMENTS IN CRISPR DIAGNOSTICS, AND GOVERNMENT FUNDING FOR THE DEVELOPMENT OF CRISPR DETECTION KITS ARE EXPECTED TO DRIVE THE ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET FROM 2022 TO 2029
FIGURE 13 CLASS SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET FROM 2022 & 2029
FIGURE 14 ASIA PACIFIC CRISPR GENE PATENT SCENARIO, BY APPLICATION
FIGURE 15 CRISPR PATENT LANDSCAPE AND NUMBER OF APPLICATIONS OF NEW PATENT FAMILIES FILED WORLDWIDE, 2001 TO 2019
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET
FIGURE 17 INCIDENCE OF VARIOUS TYPES OF CANCER IN 2020
FIGURE 18 PREVALENCE OF HUNTINGTON’S DISEASE IN 2019
FIGURE 19 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, 2021
FIGURE 20 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, 2022-2029 (USD MILLION)
FIGURE 21 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, CAGR (2022-2029)
FIGURE 22 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, LIFELINE CURVE
FIGURE 23 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, 2021
FIGURE 24 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, 2022-2029 (USD MILLION)
FIGURE 25 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, CAGR (2022-2029)
FIGURE 26 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, LIFELINE CURVE
FIGURE 27 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, 2021
FIGURE 28 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 29 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 30 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 31 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, 2021
FIGURE 32 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, 2022-2029 (USD MILLION)
FIGURE 33 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, CAGR (2022-2029)
FIGURE 34 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, LIFELINE CURVE
FIGURE 35 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, 2021
FIGURE 36 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 37 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, CAGR (2022-2029)
FIGURE 38 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 39 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 40 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 41 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 42 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SNAPSHOT (2021)
FIGURE 44 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2021)
FIGURE 45 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2029)
FIGURE 46 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2021 & 2029)
FIGURE 47 ASIA-PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS (2022 & 2029)
FIGURE 48 ASIA PACIFIC CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY SHARE 2021 (%)

研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。














