根据2020年3月发表的《生殖支原体尿道炎:流行情况和耐药模式》报告,生殖支原体是男性尿道炎的主要病因之一,一项对914名受试者进行的研究显示,28.7%的人口感染了生殖支原体(MG)。2019年2月的新闻报道,克罗恩病患者的直肠和肛管中分离出支原体,这意味着支原体也是导致胃肠道感染流行的元凶。
完整报告请访问https://databridgemarketresearch.com/reports/global-mycoplasma-testing-in-clinical-market
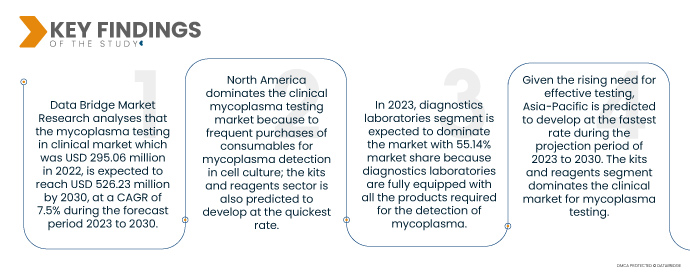
Data Bridge Market Research 分析称, 临床市场的支原体检测 2022 年为 2.9506 亿美元,预计到 2030 年将达到 5.2623 亿美元,预测期内(2023 年至 2030 年)的复合年增长率为 7.5%。开发了高灵敏度的 PCR 和 qPCR,提高了对 90 多种支原体检测的特异性。测试包括各种商品,例如读取器、测定、仪器、试剂盒和试剂,所有这些都有助于市场增长。大量市场参与者参与生产用于识别各种支原体物种的设备和耗材,为市场扩张铺平了道路。
慢性病数量不断增加 预计将推动市场增长率
当今,由于卫生条件差等各种原因,各种传染病不断增加。支原体通过侵袭身体的各个部位而引起各种疾病。支原体与尿道炎、克罗恩病、肺炎和神经系统疾病等有关。由于支原体是许多疾病的主要原因,因此为了及时提供适当的治疗,临床检测的必要性正在增加。因此,疾病数量的增加推动了全球临床市场中支原体检测的扩张。
报告范围和市场细分
|
报告指标
|
细节
|
|
预测期
|
2023 至 2030 年
|
|
基准年
|
2022
|
|
历史岁月
|
2021 (可定制为 2015 - 2020)
|
|
定量单位
|
收入(百万美元)、销量(单位)、定价(美元)
|
|
涵盖的领域
|
产品(试剂盒和试剂、仪器、服务)、技术(微生物培养技术/直接测定、聚合酶链反应、ELISA、DNA 染色/间接测定、酶法)、疾病领域(呼吸系统、泌尿生殖系统、胃肠道、肌肉骨骼、心血管、其他)、最终用户(诊断实验室、医院)
|
|
覆盖国家
|
北美洲的美国、加拿大和墨西哥、德国、法国、英国、荷兰、瑞士、比利时、俄罗斯、意大利、西班牙、土耳其、欧洲其他地区、中国、日本、印度、韩国、新加坡、马来西亚、澳大利亚、泰国、印度尼西亚、菲律宾、亚太地区 (APAC) 的其他地区、沙特阿拉伯、阿联酋、南非、埃及、以色列、中东和非洲 (MEA) 的其他地区、巴西、阿根廷和南美洲其他地区
|
|
涵盖的市场参与者
|
AB ANALITICA srl(意大利)、bioMérieux SA(法国)、ELITechGroup(法国)、Liofilchem Srl(意大利)、安捷伦科技公司(美国)、PromoCell GmbH(德国)、F. Hoffmann-La Roche Ltd(瑞士)、OSANG Healthcare(韩国)、Sacace Biotechnologies Srl(意大利)、Lonza(瑞士)、Merck KGaA(德国)、Seegene Inc.(韩国)、Clongen Laboratories, LLC(美国)、Bio-Rad Laboratories, Inc.(美国)、Charles River Laboratories(美国)、Bionique Testing Laboratories, Inc.(美国)和 ZEAKON Diagnostics(美国)
|
|
报告中涵盖的数据点
|
除了市场价值、增长率、细分市场、地理覆盖范围、市场参与者和市场情景等市场洞察外,Data Bridge 市场研究团队策划的市场报告还包括深入的专家分析、患者流行病学、渠道分析、定价分析和监管框架
|
细分分析:
全球临床支原体检测市场根据产品、技术、疾病领域和最终用户分为四个显著的部分。
- 根据产品,临床支原体检测市场细分为试剂盒和试剂、仪器和服务。2023 年,试剂盒和试剂部分预计将以 54.31% 的市场份额占据市场主导地位,因为试剂盒和试剂被反复购买作为检测支原体的产品。
- 根据技术,临床市场的支原体检测可分为微生物培养技术/直接测定、聚合酶链反应、ELISA、DNA 染色/间接测定、酶法。到 2023 年,微生物培养技术/直接测定部分预计将以 36.42% 的市场份额占据市场主导地位,因为这些技术具有成本效益,并且在获得阳性结果时具有高度特异性。
- 根据疾病领域,临床市场的支原体检测可分为呼吸系统、泌尿生殖系统、胃肠道、心血管、肌肉骨骼等。2023 年,呼吸系统疾病预计将占据市场主导地位,占比 36.37%,因为支原体会影响患有轻度至重度肺炎的儿童和成人。
呼吸系统疾病将主导临床支原体检测市场的疾病领域
呼吸系统领域将成为疾病领域中占主导地位的领域,市场份额约为 37.00%。这是因为市场中基础设施开发活动的数量不断增加,尤其是在发展中经济体中。此外,全球医疗保健行业的增长和扩张将进一步促进该领域的增长。
- 根据最终用户,临床支原体检测市场分为医院和诊断实验室。2023 年,诊断实验室预计将以 55.14% 的市场份额占据市场主导地位,因为诊断实验室配备了检测支原体所需的所有产品,并拥有熟练的专业人员。
诊断实验室部门将主导临床市场支原体检测的最终用户部分
诊断实验室中心部分将成为最终用户细分市场的主导部分。这是因为市场上诊断实验室的数量不断增加,尤其是在发展中经济体。此外,全球范围内研究开发服务的增长和扩张将进一步促进这一细分市场的增长。
主要参与者
Data Bridge Market Research 将以下公司视为主要的市场参与者:AB ANALITICA srl(意大利)、bioMérieux SA(法国)、ELITechGroup(法国)、Liofilchem Srl(意大利)、安捷伦科技公司(美国)、PromoCell GmbH(德国)、F. Hoffmann-La Roche Ltd(瑞士)、OSANG Healthcare(韩国)、Sacace Biotechnologies Srl(意大利)、Lonza(瑞士)、Merck KGaA(德国)、Seegene Inc.(韩国)、Clongen Laboratories, LLC(美国)、Bio-Rad Laboratories, Inc.(美国)、Charles River Laboratories(美国)、Bionique Testing Laboratories, Inc.(美国)和 ZEAKON Diagnostics(美国)。
市场发展
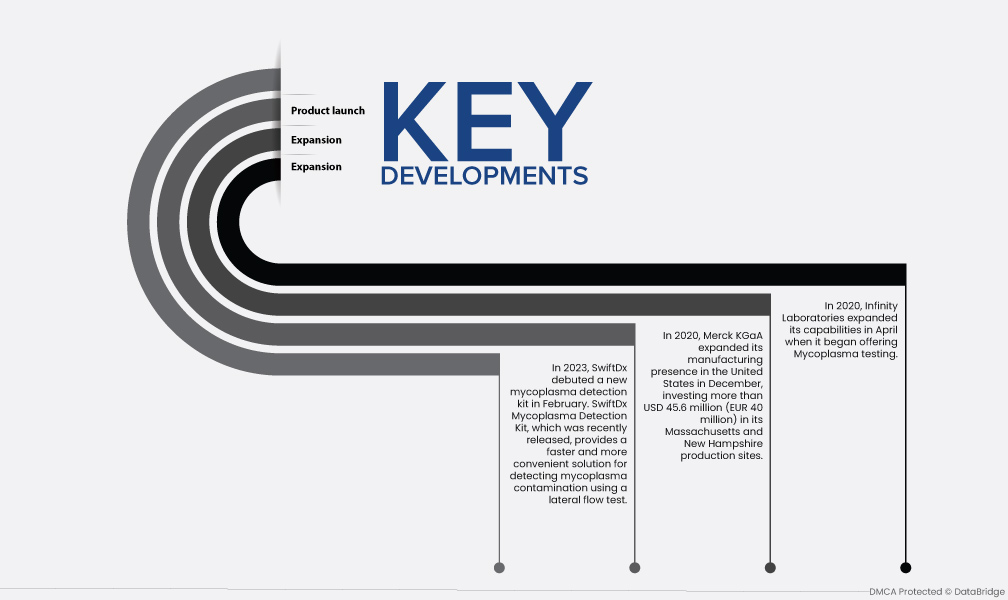
- 2023年,SwiftDx于2月推出了一款新型支原体检测试剂盒。最近发布的SwiftDx支原体检测试剂盒为使用横向流动测试检测支原体污染提供了更快速、更便捷的解决方案。
- 2020 年 12 月,默克集团扩大了其在美国的生产业务,在其马萨诸塞州和新罕布什尔州的生产基地投资超过 4560 万美元(4000 万欧元)。这些基地旨在生产各种生物制药产品,同时提高公司的生产能力。
- 2020 年 4 月,Infinity Laboratories 开始提供支原体检测,扩大了其能力。随着扩张、合作、收购等关键战略的实施,顶级市场参与者的能力将得到提高,从而推动市场扩张。
区域分析
从地理上看,市场报告涵盖的国家包括北美洲的美国、加拿大和墨西哥、欧洲的德国、法国、英国、荷兰、瑞士、比利时、俄罗斯、意大利、西班牙、土耳其、欧洲其他地区、亚太地区 (APAC) 的中国、日本、印度、韩国、新加坡、马来西亚、澳大利亚、泰国、印度尼西亚、菲律宾、亚太地区 (APAC) 的其他地区、沙特阿拉伯、阿联酋、南非、埃及、以色列、中东和非洲 (MEA) 的其他地区、南美洲的巴西、阿根廷和南美洲其他地区。
根据 Data Bridge 市场研究分析:
北美是临床支原体检测领域的主要地区 市场 在 2023 年至 2030 年的预测期内
北美占据临床支原体检测市场的主导地位,因为经常购买用于细胞培养中支原体检测的消耗品;预计试剂盒和试剂行业也将以最快的速度发展。
亚太地区预计将成为 临床中的支原体检测 市场 在 2023 年至 2030 年的预测期内
鉴于对有效检测的需求不断增长,预计亚太地区将在 2023 年至 2030 年的预测期内以最快的速度发展。试剂盒和试剂部分在支原体检测的临床市场中占据主导地位。
有关临床市场支原体检测的更多详细信息 报告,请点击这里 – https://www.databridgemarketresearch.com/reports/global-mycoplasma-testing-in-clinical-market