Global Lal Testing Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
229.94 Million
USD
458.18 Million
2024
2032
USD
229.94 Million
USD
458.18 Million
2024
2032
| 2025 –2032 | |
| USD 229.94 Million | |
| USD 458.18 Million | |
|
|
|
|
Сегментация мирового рынка LAL-тестирования по методам тестирования (тест на эндотоксин в гель-тромбе, хромогенный тест на эндотоксин и турбидиметрический тест на эндотоксин), применение (производство медицинских приборов и фармацевтическое производство) — тенденции отрасли и прогноз до 2032 г.
Каковы размер и темпы роста мирового рынка LAL-тестирования?
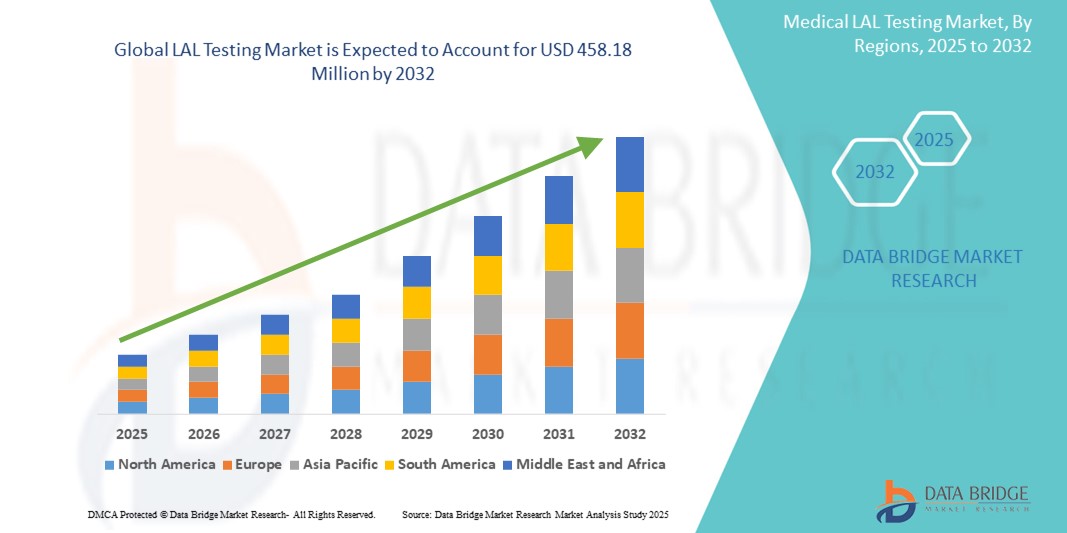
- Объем мирового рынка LAL-тестирования оценивался в 229,94 млн долларов США в 2024 году и, как ожидается , достигнет 458,18 млн долларов США к 2032 году при среднегодовом темпе роста 9,00% в течение прогнозируемого периода.
- Ожидается, что в ближайшие годы на мировом рынке LAL-тестирования произойдет значительный рост, обусловленный такими факторами, как рост распространенности инфекционных заболеваний, растущая обеспокоенность относительно безопасности фармацевтической продукции и медицинских устройств, а также растущее внедрение LAL-тестирования в различных отраслях промышленности.
Каковы основные выводы рынка LAL-тестирования?
- Фармацевтическая промышленность вносит большой вклад в рост рынка, поскольку LAL-тестирование является важнейшим шагом в обеспечении безопасности и качества лекарственных средств. Кроме того, отрасль медицинских приборов все чаще использует LAL-тестирование для соответствия нормативным требованиям и повышения безопасности продукции
- Северная Америка доминировала на рынке LAL-тестирования с наибольшей долей выручки в 42,8% в 2024 году, что обусловлено строгими нормативными требованиями к тестированию на эндотоксины в фармацевтической и медицинской промышленности.
- Прогнозируется, что рынок LAL-тестирования в Азиатско-Тихоокеанском регионе будет расти самыми быстрыми темпами в 12,6% в год в период с 2025 по 2032 год, что обусловлено быстрым расширением фармацевтического производства, особенно в таких странах, как Китай, Индия и Япония.
- Сегмент гель-тромб-тестов на эндотоксины доминировал на рынке с наибольшей долей выручки на рынке в 48,6% в 2024 году благодаря его широкому принятию регулирующими органами, экономической эффективности и простоте качественного обнаружения эндотоксинов.
Область применения отчета и сегментация рынка LAL-тестирования
|
Атрибуты |
Ключевые рыночные данные по тестированию LAL |
|
Охваченные сегменты |
|
|
Страны, охваченные |
Северная Америка
Европа
Азиатско-Тихоокеанский регион
Ближний Восток и Африка
Южная Америка
|
|
Ключевые игроки рынка |
|
|
Возможности рынка |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, анализ цен, анализ доли бренда, опрос потребителей, демографический анализ, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья/расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Какова основная тенденция на рынке LAL-тестирования?
« Достижения в области биотехнологии и переход к рекомбинантному LAL-тестированию »
- Значительной и развивающейся тенденцией на мировом рынке LAL-тестирования является растущий сдвиг в сторону рекомбинантных альтернатив LAL и биотехнологических инноваций, обусловленный проблемами устойчивости и нормативной поддержкой. Растущий спрос на тестирование эндотоксинов, особенно в фармацевтической и медицинской промышленности, ускоряет принятие синтетических и неживотных решений для LAL-тестирования
- Например, компания Lonza (Швейцария) представила анализ на рекомбинантный фактор C (rFC) PyroGene, который является устойчивой и надежной альтернативой традиционному тестированию LAL, снижая зависимость от крови мечехвоста и обеспечивая при этом соблюдение нормативных требований в области контроля качества.
- Растущая регулирующая поддержка со стороны таких организаций, как FDA США и Европейская фармакопея, продвигающих альтернативные методы обнаружения эндотоксинов, подталкивает производителей фармацевтической продукции к внедрению передовых технологий тестирования, которые соответствуют как стандартам эффективности, так и этическим стандартам.
- Рекомбинантные методы тестирования LAL смягчают экологические проблемы и повышают точность, последовательность партий и снижают риски в цепочке поставок, связанные с добычей мечехвостов. На рынке наблюдается более широкое применение таких технологий в производстве биопрепаратов, вакцин и инъекционных препаратов
- Эта тенденция трансформирует ландшафт обнаружения эндотоксинов, поскольку такие компании, как Charles River Laboratories (Великобритания) и Thermo Fisher Scientific (США), инвестируют в масштабируемые платформы LAL-тестирования нового поколения, которые поддерживают строгие требования к контролю качества в фармацевтическом и биотехнологическом секторах.
- По мере того, как отрасль внедряет инновации, ожидается, что спрос на рекомбинантные, надежные и экологически безопасные решения для LAL-тестирования резко возрастет, особенно в регионах с развитым фармацевтическим производством, таких как Северная Америка, Европа и Азиатско-Тихоокеанский регион.
Каковы основные движущие силы рынка LAL-тестирования?
- Растущая распространенность инъекционных препаратов, вакцин и биофармацевтических продуктов является основным фактором, обусловливающим растущий спрос на LAL-тестирование, учитывая его важную роль в обеспечении стерильности продукции и безопасности пациентов.
- Например, в феврале 2024 года компания WuXi AppTec (Китай) расширила свои возможности по тестированию эндотоксинов, чтобы удовлетворить растущий мировой спрос на стерильные биопрепараты, расширив комплексный портфель услуг компании по контролю качества. Такие разработки стимулируют рост отрасли
- Строгие нормативные требования таких органов, как FDA США, EMA и фармакопейные организации, требуют регулярного тестирования на эндотоксины фармацевтических препаратов, медицинских приборов и биологических продуктов, обеспечивая постоянный спрос на рынке.
- Рост расходов на здравоохранение во всем мире, расширение фармацевтических НИОКР и постоянный спрос на высокочистые инъекционные препараты, включая моноклональные антитела, клеточную и генную терапию, а также вакцины против COVID-19, еще больше усиливают потребность в надежных решениях для LAL-тестирования.
- Кроме того, технологические достижения, ведущие к более быстрым, чувствительным и автоматизированным LAL-тестам, повышают эффективность работы и сокращают время выполнения тестов, способствуя широкому внедрению в лабораториях контроля качества.
Какой фактор препятствует росту рынка LAL-тестирования?
- Чрезмерная зависимость от крови мечехвоста для традиционного LAL-тестирования остается серьезной экологической и этической проблемой, приводящей к ограничению поставок и контролю со стороны регулирующих органов, что может повлиять на стабильность рынка.
- Например, опасения по поводу популяции мечехвостов вдоль атлантического побережья США вызвали дебаты среди природоохранных организаций, регулирующих органов и заинтересованных сторон отрасли, что подчеркивает необходимость альтернативных методов тестирования.
- Более того, несмотря на технологический прогресс, ограниченная глобальная осведомленность и более медленное нормативное принятие рекомбинантных альтернатив LAL на некоторых рынках продолжают ограничивать широкое использование. Нормативная гармонизация в регионах все еще развивается, создавая фрагментированные пути одобрения
- Высокие первоначальные инвестиционные затраты на современные платформы обнаружения эндотоксинов и необходимость в технической экспертизе также могут стать препятствиями, особенно для малых и средних фармацевтических производителей на развивающихся рынках.
- Чтобы преодолеть эти проблемы, компании должны сосредоточиться на повышении осведомленности о вариантах устойчивого тестирования, сотрудничать с регулирующими органами для ускорения глобальной гармонизации и инвестировать в экономически эффективные, масштабируемые решения для расширения доступа к рынку.
Как сегментирован рынок LAL-тестирования?
Рынок сегментирован на основе методов тестирования и применения.
- По методам тестирования
На основе методов тестирования рынок LAL-тестирования сегментирован на тест на эндотоксины с гель-тромбой, хромогенный тест на эндотоксины и турбидиметрический тест на эндотоксины. Сегмент теста на эндотоксины с гель-тромбой доминировал на рынке с наибольшей долей выручки рынка в 48,6% в 2024 году благодаря его широкому принятию регулирующими органами, экономической эффективности и простоте качественного обнаружения эндотоксинов. Производители фармацевтической продукции и компании, производящие медицинские устройства, часто полагаются на тесты на эндотоксины с гель-тромбой из-за их проверенной репутации, простоты использования и пригодности для рутинного тестирования партий при выпуске в стерильных производственных условиях. Минимальные требования к оборудованию для этого метода делают его особенно популярным среди малых и средних производителей и для первоначального скрининга продукции.
Ожидается, что сегмент хромогенных тестов на эндотоксины будет демонстрировать самый быстрый CAGR с 2025 по 2032 год, что обусловлено растущим спросом на количественное, высокочувствительное обнаружение эндотоксинов в биопрепаратах, вакцинах и инъекционных фармацевтических препаратах. Хромогенные тесты предлагают точное измерение эндотоксинов в реальном времени, поддерживая более строгие стандарты контроля качества в высокочистых производственных средах. Растущая сложность передовых методов лечения также стимулирует внедрение этого метода в фармацевтической и биотехнологической отраслях.
- По применению
На основе сферы применения рынок LAL-тестирования сегментируется на производство медицинских приборов и фармацевтическое производство. На сегмент фармацевтического производства в 2024 году пришлась наибольшая доля выручки рынка в размере 65,4%, что обусловлено строгими мировыми нормативными требованиями к тестированию эндотоксинов в инъекционных препаратах, биопрепаратах, вакцинах и парентеральных терапиях. Рост производства биопрепаратов, моноклональных антител, клеточной и генной терапии требует надежного контроля эндотоксинов, что делает LAL-тестирование неотъемлемой частью обеспечения качества фармацевтической продукции.
Ожидается, что сегмент производства медицинских устройств зарегистрирует самые быстрые темпы роста с 2025 по 2032 год, поскольку усиление контроля со стороны регулирующих органов требует проведения испытаний на эндотоксины для имплантируемых устройств, хирургических инструментов и других критически важных медицинских изделий. Повышенный спрос на стерильные, высококачественные медицинские устройства, особенно в ортопедии, кардиологии и неврологии, стимулирует внедрение LAL-тестирования в этом секторе. Резкий рост глобальной инфраструктуры здравоохранения еще больше ускоряет рост рынка в приложениях для производства устройств.
Какой регион занимает наибольшую долю рынка LAL-тестирования?
- Северная Америка доминировала на рынке LAL-тестирования с наибольшей долей выручки в 42,8% в 2024 году, что обусловлено строгими нормативными требованиями к тестированию на эндотоксины в фармацевтической и медицинской промышленности.
- Регион выигрывает за счет хорошо развитого биофармацевтического сектора, повышенного внимания к безопасности пациентов и значительных инвестиций в контроль качества инъекционных препаратов и имплантируемых медицинских устройств.
- Спрос на LAL-тестирование дополнительно поддерживается растущим портфелем биологических препаратов, ростом активности клинических испытаний и строгими требованиями FDA США к тестированию на эндотоксины, что делает Северную Америку мировым лидером в этой области.
Обзор рынка LAL-тестирования в США
Рынок LAL-тестирования в США получил наибольшую долю выручки в 78% в Северной Америке в 2024 году, что обусловлено доминирующим в стране сектором фармацевтического производства и ведущими мировыми центрами биотехнологических инноваций. Рост производства биопрепаратов, вакцин и стерильных инъекционных препаратов усилил спрос на точные методы обнаружения эндотоксинов. Более того, строгие нормативные рамки от FDA США и USP для тестирования пирогенов и эндотоксинов продолжают подпитывать рост рынка. Мощная отрасль медицинских устройств в стране и всплеск хирургических процедур также способствуют принятию LAL-тестирования.
Обзор европейского рынка LAL-тестирования
Ожидается, что рынок LAL-тестирования в Европе будет расширяться со значительным среднегодовым темпом роста в течение прогнозируемого периода, поддерживаемого строгим регулирующим надзором со стороны таких организаций, как Европейское агентство по лекарственным средствам (EMA), и ростом лекарственных средств передовой терапии (ATMP). Растущая разработка биологических препаратов в сочетании с фокусом региона на фармацевтическом контроле качества ускоряет спрос на LAL-тестирование. Кроме того, инициативы, направленные на снижение рисков заражения в медицинских учреждениях, и сильная база по производству медицинских приборов способствуют расширению рынка как на устоявшихся, так и на развивающихся европейских рынках.
Обзор рынка тестирования LAL в Великобритании
Рынок LAL-тестирования в Великобритании, как ожидается, будет расти в заметном среднегодовом темпе, поддерживаемом активной фармацевтической экосистемой НИОКР в стране, передовыми возможностями биопроизводства и соответствием нормативных требований международным стандартам контроля эндотоксинов. Растущее производство биопрепаратов, вакцин и клеточной терапии увеличивает спрос на LAL-тестирование. Кроме того, растущий акцент на безопасности здравоохранения и предотвращении заражения в больницах и лабораториях еще больше стимулирует рынок.
Обзор рынка тестирования LAL в Германии
Рынок LAL-тестирования в Германии готов к устойчивому росту, обусловленному его статусом одного из ведущих европейских центров фармацевтического производства, инноваций в области медицинских приборов и исследований в области биотехнологий. Повышенный спрос на стерильные инъекционные препараты и имплантируемые устройства в сочетании с сильной культурой соблюдения нормативных требований в Германии подпитывает внедрение LAL-тестирования. Стремление страны к высокотехнологичным решениям в области здравоохранения и растущее внимание к производству биологических препаратов также способствуют расширению рынка.
Какой регион является самым быстрорастущим на рынке LAL-тестирования?
Прогнозируется, что рынок LAL-тестирования в Азиатско-Тихоокеанском регионе будет расти с самым быстрым среднегодовым темпом роста в 12,6% с 2025 по 2032 год, что обусловлено быстрым расширением фармацевтического производства, особенно в таких странах, как Китай, Индия и Япония. Рост инвестиций региона в биотехнологии, рост экспорта инъекционных препаратов и медицинских устройств, а также развитие нормативных стандартов для тестирования эндотоксинов являются ключевыми факторами роста. Расширение инфраструктуры здравоохранения в Азиатско-Тихоокеанском регионе и фокус на внутреннем производстве вакцин также подпитывают спрос на LAL-тестирование, особенно после пандемии.
Обзор рынка LAL-тестирования в Японии
Рынок LAL-тестирования в Японии набирает обороты, подпитываемый надежным фармацевтическим производством страны, высокими стандартами качества здравоохранения и фокусом на передовых методах лечения. Растущий спрос на стерильные инъекционные продукты в сочетании с государственной поддержкой биопрепаратов и регенеративной медицины расширяет применение LAL-тестирования. Акцент Японии на безопасности пациентов в сочетании с инновациями в области контроля загрязнения позиционирует рынок для устойчивого роста.
Обзор рынка тестирования LAL в Китае
На китайский рынок LAL-тестирования пришлась наибольшая доля выручки в Азиатско-Тихоокеанском регионе в 2024 году, чему способствовало бурно развивающееся фармацевтическое производство в стране, растущая биотехнологическая отрасль и увеличивающийся экспорт инъекционных лекарств и медицинских приборов. Строгие государственные правила безопасности продукции, стремление к самообеспечению в производстве вакцин и биопрепаратов и значительные инвестиции в НИОКР в сфере здравоохранения продолжают стимулировать спрос на надежные методы тестирования эндотоксинов, такие как LAL-тестирование, по всему Китаю.
Какие компании являются ведущими на рынке LAL-тестирования?
Индустрию LAL-тестирования в основном возглавляют известные компании, в том числе:
- Pacific BioLabs (США)
- Лонза (Швейцария)
- Nelson Laboratories, LLC (США)
- Bio-Synthesis Inc (США)
- Биогеникс (Индия)
- GenScript (США)
- Thermo Fisher Scientific, Inc. (США)
- SGS Société Générale de Surveillance SA (Швейцария)
- WuXi AppTec (Китай)
- Сарториус АГ (Германия)
- АстраЗенека (Великобритания)
- Новасеп (Франция)
- Merck KGaA (Германия)
- Charles River Laboratories (Великобритания)
Каковы последние события на мировом рынке LAL-тестирования?
- В декабре 2024 года Ellab завершила приобретение PharmaProcess в Италии и Швейцарии с целью укрепления своего портфеля услуг в области естественных наук. Интеграция объединяет нормативные знания PharmaProcess с решениями Ellab по обеспечению соответствия, обеспечивая комплексную поддержку фармацевтическим и биотехнологическим компаниям в обеих странах. Ожидается, что этот шаг расширит региональное присутствие Ellab и повысит возможности обслуживания для требований к эндотоксинам и качеству тестирования
- В сентябре 2024 года Lonza Walkersville инициировала расширение своего производственного участка по анализу эндотоксинов в Уокерсвилле, штат Мэриленд, с модернизацией объекта площадью 18 000 квадратных футов. Это расширение призвано увеличить производственные мощности в ответ на растущий мировой спрос на анализы эндотоксинов, необходимые для обеспечения безопасности инъекционных препаратов и медицинских устройств. Развитие еще больше укрепит позиции Lonza на рынке LAL-тестирования
- В июне 2024 года компания FUJIFILM Wako Pure Chemicals представила два передовых решения для обнаружения эндотоксинов и пирогенов: набор для обнаружения пирогенов LumiMAT, тест нового поколения на активацию моноцитов, и PYROSTAR Neo+, рекомбинантный белковый реагент для тестирования бактериальных эндотоксинов, который стал доступен по всему миру в июле 2024 года. Эти инновации повышают точность и эффективность обнаружения эндотоксинов, поддерживая стандарты безопасности в фармацевтических и медицинских приложениях.
- В марте 2024 года компания AmeboGenesis достигла значительного прорыва в устойчивом производстве амебоцитов, внедрив новаторскую технологию, которая позволяет производить в лабораторных условиях биоидентичные амебоциты для тестирования LAL, устраняя необходимость сбора крови мечехвоста. Поскольку кровь мечехвоста имеет решающее значение для производства лизата амебоцитов Limulus (LAL), это достижение способствует устойчивости, одновременно обеспечивая безопасность инъекционных препаратов, вакцин и медицинских устройств по всему миру.
- В октябре 2023 года компания Lonza запустила две системы теста активации моноцитов (МАТ), PyroCell MAT Human Serum (HS) Rapid System и PyroCell MAT Rapid System, разработанные для более быстрого и надежного тестирования пирогенов для производителей фармацевтической продукции. Эти системы повышают эффективность обнаружения пирогенов, способствуя повышению безопасности и соблюдению нормативных требований в процессах производства лекарственных препаратов и медицинских изделий.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.