Global Influenza Drug Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
981.68 Billion
USD
1,168.36 Billion
2024
2032
USD
981.68 Billion
USD
1,168.36 Billion
2024
2032
| 2025 –2032 | |
| USD 981.68 Billion | |
| USD 1,168.36 Billion | |
|
|
|
|
Сегментация мирового рынка лекарств от гриппа по типу (грипп A, грипп B и грипп C), лечению (вакцины и лекарства), способу введения (перорально, внутримышечно, внутрикожно, интраназально и внутривенно), возрасту (дети и взрослые), конечному пользователю (больницы и уход на дому), каналу сбыта (прямые тендеры и розничные продажи) — тенденции отрасли и прогноз до 2032 г.
Размер рынка лекарств от гриппа
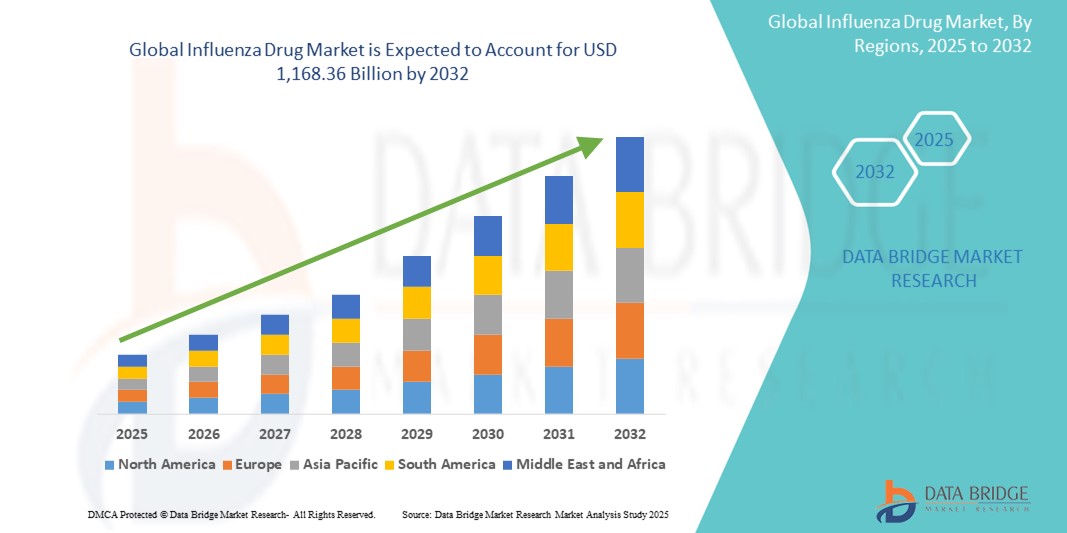
- Объем мирового рынка противогриппозных препаратов оценивался в 981,68 млрд долларов США в 2024 году и, как ожидается , достигнет 1 168,36 млрд долларов США к 2032 году при среднегодовом темпе роста 2,20% в течение прогнозируемого периода.
- Рост рынка во многом обусловлен ростом заболеваемости сезонным гриппом и другими инфекционными респираторными заболеваниями, а также увеличением числа пациентов, подверженных риску развития осложнений, связанных с гриппом.
- Кроме того, растущие инвестиции в научно-исследовательскую деятельность фармацевтических компаний и научно-исследовательских институтов с целью разработки современных и усовершенствованных лекарственных средств, включая новые противовирусные препараты и рекомбинантные вакцины , ускоряют внедрение лекарственных средств против гриппа, тем самым значительно стимулируя рост отрасли.
Анализ рынка лекарств от гриппа
- Мировой рынок противогриппозных препаратов охватывает целый ряд противовирусных препаратов и вакцин, предназначенных для профилактики, лечения и облегчения симптомов гриппозных инфекций, которые представляют собой серьезную проблему для общественного здравоохранения из-за их рецидивирующего и сезонного характера, постоянных вирусных мутаций и потенциальной возможности возникновения пандемий.
- Растущий спрос на противогриппозные препараты обусловлен в первую очередь устойчивой глобальной распространенностью гриппозных инфекций, растущей осведомленностью о преимуществах вакцинации и постоянными достижениями в фармацевтических исследованиях, ведущими к созданию более эффективных противовирусных препаратов и вакцин следующего поколения.
- Северная Америка доминирует на рынке лекарств от гриппа с самой большой долей выручки в 60,5% в 2024 году, характеризуется надежной инфраструктурой здравоохранения, высоким уровнем охвата вакцинацией и присутствием крупных фармацевтических компаний. В США наблюдается сильный рост рынка, обусловленный обширными кампаниями в области общественного здравоохранения, правительственными инициативами по готовности к пандемии и продолжающимися НИОКР в области новых методов лечения гриппа и вакцин
- Ожидается, что Азиатско-Тихоокеанский регион станет самым быстрорастущим регионом на рынке противогриппозных препаратов в прогнозируемый период из-за повышения осведомленности о гриппе, увеличения расходов на здравоохранение, растущей урбанизации и большого количества пациентов, восприимчивых к инфекциям, в густонаселенных странах.
- Сегмент гриппа А доминирует на рынке лекарств от гриппа с долей рынка 47,78% в 2024 году, что обусловлено более высокой скоростью мутаций, более широким кругом хозяев, значительным пандемическим потенциалом и повышенной тяжестью заболевания, что требует более сложных стратегий вакцинации и частого обновления методов лечения.
Область применения отчета и сегментация рынка противогриппозных препаратов
|
Атрибуты |
Основные сведения о рынке лекарств от гриппа |
|
Охваченные сегменты |
|
|
Страны, охваченные |
Северная Америка
Европа
Азиатско-Тихоокеанский регион
Ближний Восток и Африка
Южная Америка
|
|
Ключевые игроки рынка |
|
|
Возможности рынка |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, анализ цен, анализ доли бренда, опрос потребителей, демографический анализ, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья/расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции рынка лекарств от гриппа
«Растущее внедрение телемедицины и цифровых инструментов здравоохранения»
- Значительная и ускоряющаяся тенденция на мировом рынке лекарств от гриппа — это растущая интеграция телемедицины и цифровых медицинских инструментов для управления гриппом, диагностики и назначения лекарств. Это слияние технологий значительно повышает доступность медицинских услуг, особенно для пациентов с гриппом
- Например, виртуальные консультации позволяют людям с симптомами гриппа связаться с медицинскими работниками, не выходя из дома, что снижает риск передачи вируса в клиниках и больницах. Телемедицинские платформы могут облегчить быструю диагностику на основе оценки симптомов и направлять пациентов на надлежащий самостоятельный уход или назначение противовирусных препаратов, таких как осельтамивир или балоксавир. Примерно 33% систем здравоохранения интегрируют лечение гриппа в телемедицинские услуги, что повышает доступность в сельских регионах
- Цифровые медицинские инструменты, включая мобильные приложения и носимые устройства, позволяют осуществлять непрерывный мониторинг физиологических параметров, которые могут указывать на начало или прогрессирование гриппа. Некоторые умные термометры , например, от KINSA, могут отслеживать совокупные данные о температуре для выявления потенциальных очагов гриппа. Эти технологии могут предоставлять ранние оповещения, побуждая людей раньше обращаться за медицинской помощью, что имеет решающее значение для эффективности противовирусного лечения
- Бесшовная интеграция цифровых медицинских инструментов с системами здравоохранения также поддерживает более широкие усилия по надзору за общественным здоровьем. Данные с носимых устройств могут способствовать надзору за гриппом в режиме реального времени, помогая органам здравоохранения отслеживать вспышки и более эффективно распределять ресурсы. Это улучшенное наблюдение может информировать о целевых кампаниях вакцинации и своевременном распределении противовирусных препаратов
- Эта тенденция к более доступному, проактивному и основанному на данных управлению гриппом фундаментально меняет взаимодействие с пациентами и предоставление медицинских услуг. Следовательно, фармацевтические компании и поставщики медицинских услуг изучают партнерские отношения и разрабатывают платформы, которые используют эти цифровые возможности для улучшения результатов лечения пациентов и соблюдения режима приема лекарств.
- Спрос на противогриппозные препараты косвенно повышается благодаря этим тенденциям, поскольку телемедицина облегчает пациентам получение своевременных рецептов, а цифровой мониторинг может привести к более ранней диагностике и вмешательству, максимально повышая эффективность доступных методов лечения.
Динамика рынка противогриппозных препаратов
Водитель
«Растущая потребность в связи с устойчивой глобальной распространенностью гриппа и инициативами в области общественного здравоохранения»
- Растущая и устойчивая глобальная распространенность сезонных эпидемий гриппа в сочетании с сохраняющейся угрозой пандемических штаммов и активными инициативами в области общественного здравоохранения являются существенным фактором повышенного спроса на противогриппозные препараты и вакцины.
- Например, правительства по всему миру, включая США, Европу и Азию, постоянно запускают масштабные кампании вакцинации и инвестируют в национальные программы иммунизации, чтобы смягчить воздействие ежегодных сезонов гриппа и подготовиться к потенциальным пандемиям. Эти инициативы, наряду с усилением надзора и быстрыми достижениями в диагностике, приводят к большему стимулированию вакцинации и раннего лечения противовирусными препаратами
- По мере того, как растет осведомленность населения и поставщиков медицинских услуг о серьезности гриппа и его потенциальных осложнениях, растет спрос на профилактические и терапевтические решения. Это особенно актуально для групп высокого риска, таких как пожилые люди, маленькие дети и люди с сопутствующими заболеваниями
- Кроме того, постоянные исследования и разработки фармацевтических компаний с целью создания более эффективных и более широкого спектра противовирусных препаратов, а также вакцин нового поколения расширяют спектр доступных методов лечения и повышают эффективность существующих вмешательств, тем самым способствуя росту рынка.
- Необходимость в обновлении формул вакцин каждый год из-за постоянно меняющейся природы вирусов гриппа обеспечивает устойчивый и повторяющийся спрос на новые продукты. Этот непрерывный цикл разработки, производства и распространения является ключевым фактором, продвигающим рынок лекарств от гриппа как в развитых, так и в развивающихся экономиках.
Сдержанность/Вызов
«Проблемы вирусной изменчивости и устойчивости к лекарствам, а также высокие затраты на разработку»
- Значительной проблемой для мирового рынка противогриппозных препаратов является присущая вирусам гриппа изменчивость, которая постоянно подвергается антигенному дрейфу и сдвигу. Эта быстрая эволюция может сделать существующие вакцины менее эффективными и привести к возникновению устойчивости к противовирусным препаратам, что представляет постоянную угрозу общественному здравоохранению
- Например, частая необходимость ежегодного обновления штаммов вакцин требует непрерывного цикла исследований, разработок и производства, что является как трудоемким, так и дорогостоящим. Кроме того, появление штаммов, устойчивых к лекарствам, например, устойчивых к старым противовирусным препаратам, таким как амантадин, или, в последнее время, некоторые вирусы H1N1 , демонстрирующие устойчивость к осельтамивиру (Тамифлю), ограничивает возможности лечения и усложняет клиническое ведение
- Решение этих биологических проблем требует значительных и постоянных инвестиций в НИОКР для новых противовирусных агентов с новыми механизмами действия и более широким спектром активности, а также универсальных вакцин против гриппа, которые могут обеспечить длительную защиту от нескольких штаммов. Однако разработка вакцин и лекарств является исключительно длительным и дорогостоящим процессом, часто занимающим 10-15 лет и обходящимся в сотни миллионов и более миллиарда долларов США, с высоким процентом неудач
- Кроме того, логистические проблемы, особенно поддержание «холодовой цепи» для распространения вакцин от производственных площадок до отдаленных районов, могут привести к потерям и снижению эффективности. Такие факторы, как неадекватная инфраструктура, человеческие ошибки и перебои с электроснабжением в странах с низким уровнем дохода, создают значительные препятствия
- Высокая начальная стоимость разработки и вывода на рынок новых лекарств и вакцин от гриппа в сочетании со строгими процессами одобрения регулирующими органами создает значительные препятствия для производителей. Это часто приводит к более высоким ценам на передовые методы лечения, что потенциально ограничивает доступ на рынках, чувствительных к цене, или для недостаточно застрахованных групп населения
- Преодоление этих проблем посредством международного сотрудничества, увеличения государственного и частного финансирования НИОКР, оптимизации нормативных схем и инвестиций в надежные глобальные распределительные инфраструктуры будет иметь решающее значение для устойчивого роста рынка и эффективной готовности к пандемии.
Масштаб рынка лекарств от гриппа
Рынок сегментирован по типу, лечению, способу введения, возрасту, конечному потребителю и каналу сбыта.
- По типу
На основе типа рынок лекарств от гриппа сегментирован на грипп А, грипп В и грипп С. Сегмент гриппа А доминирует на рынке с долей рынка 47,78% в 2024 году, что обусловлено его более высокой скоростью мутаций, более широким кругом хозяев, значительным пандемическим потенциалом и повышенной тяжестью заболевания, что требует более сложных стратегий вакцинации для контроля вспышек. Постоянная угроза штаммов гриппа А, таких как H1N1 и H3N2, обеспечивает устойчивый спрос на соответствующие препараты и вакцины
Ожидается, что грипп B будет демонстрировать самый высокий среднегодовой темп роста на рынке из-за его включения в четырехвалентные вакцины, которые обеспечивают более широкую защиту от штаммов A и B. Растущая осведомленность о роли гриппа B в сезонных эпидемиях и растущий акцент на комплексных стратегиях профилактики гриппа стимулируют спрос на более эффективные вакцины, которые охватывают эти штаммы, особенно среди уязвимых групп населения.
- По лечению
На основе лечения рынок лекарств от гриппа сегментируется на вакцины и лекарства. Сегмент вакцин доминирует на рынке с долей рынка 87,23% в 2024 году из-за его ключевой роли в профилактике заболеваний, снижении передачи и решении проблем общественного здравоохранения, связанных с сезонными вспышками и потенциальными пандемиями. Постоянное развитие технологий вакцин, включая разработку четырехвалентных вакцин, обеспечивающих более широкую защиту, еще больше укрепляет их лидерство на рынке
Сегмент лекарственных препаратов, включающий противовирусные препараты, такие как осельтамивир и балоксавир, играет решающую роль в лечении уже существующих инфекций, при этом балоксавир марбоксил (Ксофлюза) отмечен как наиболее быстрорастущий тип лекарственных средств благодаря его однократному приему и эффективности.
- По способу введения
На основе пути введения рынок противогриппозных препаратов сегментируется на пероральные, внутримышечные, внутрикожные, интраназальные и внутривенные. Ожидается, что пероральный сегмент будет доминировать на рынке с долей рынка 45,04% в 2024 году, в первую очередь за счет его удобства, простоты самостоятельного приема и широкой доступности для пациентов как в амбулаторных условиях, так и в домашних условиях. Пероральные противовирусные препараты часто являются первой линией лечения гриппа
Ожидается, что интраназальный сегмент станет самым быстрорастущим путем введения в прогнозируемый период. Это объясняется его неинвазивным, безыгольным введением, что улучшает соблюдение пациентами режима лечения, особенно в педиатрической популяции, а также его способностью вызывать как системный, так и мукозальный иммунитет
- По возрасту
Рынок противогриппозных препаратов сегментирован по возрасту на педиатрию и взрослых. Сегмент для взрослых является крупнейшим на рынке противогриппозных препаратов, с долей 66,7% в 2024 году, что обусловлено повышенным уровнем вакцинации среди пожилых людей и лиц с хроническими заболеваниями, которые подвержены более высокому риску тяжелых осложнений гриппа
Педиатрический сегмент, как ожидается, будет расти значительными среднегодовыми темпами, поскольку маленькие дети, особенно в возрасте до 5 лет, подвержены более высокому риску серьезных осложнений гриппа, что ускоряет рост рынка вакцин, подходящих для детей, и усилий по вакцинации.
- По конечному использованию
На основе конечного пользователя рынок противогриппозных препаратов сегментирован на больницы и домашний уход. Сегмент больниц доминирует на мировом рынке противогриппозных препаратов с долей рынка 72,62% в 2024 году, во многом благодаря их роли в лечении тяжелых случаев гриппа, требующих интенсивного мониторинга, внутривенных противовирусных препаратов и интенсивной терапии для пациентов с высоким риском. Больницы также служат ключевыми точками для кампаний по вакцинации
Ожидается, что сегмент домашнего ухода будет демонстрировать самые быстрые среднегодовые темпы роста (CAGR) с 2025 по 2032 год на мировом рынке лекарств от гриппа, что обусловлено растущим предпочтением вариантов домашнего лечения, достижениями в области телемедицины и растущим принятием услуг домашнего здравоохранения. Эти факторы позволяют пациентам эффективно справляться с симптомами гриппа в комфортных домашних условиях, что снижает необходимость в госпитализации и минимизирует воздействие медицинских учреждений.
- По каналу распространения
На основе канала сбыта глобальный рынок противогриппозных препаратов сегментируется на прямые тендеры и розничные продажи. Прогнозируется, что сегмент прямых тендеров будет доминировать на мировом рынке противогриппозных препаратов с наибольшей долей рынка в 56,34% в 2024 году, в первую очередь за счет крупномасштабных закупок вакцин и противовирусных препаратов правительствами и организациями общественного здравоохранения для национальных программ иммунизации и стратегических запасов. Этот канал обеспечивает оптовые закупки и эффективное распределение для кампаний массовой вакцинации.
Сегмент розничных продаж также является доминирующим каналом, выступая в качестве основных точек доступа как к вакцинам, так и к безрецептурным и рецептурным препаратам для широких слоев населения, и, как ожидается, продемонстрирует значительный рост, особенно за счет интернет-аптек из-за удобства и доступности.
Региональный анализ рынка противогриппозных препаратов
- Северная Америка доминирует на рынке противогриппозных препаратов с наибольшей долей выручки в 60,5% в 2024 году, что обусловлено надежной инфраструктурой здравоохранения, высокими показателями охвата вакцинацией и присутствием крупных фармацевтических компаний.
- Потребители в регионе уделяют большое внимание профилактической медицине и легко получают доступ к вакцинам и противовирусным препаратам.
- Широкое распространение этих препаратов также подкрепляется благоприятной политикой возмещения расходов, технологически развитым населением и растущим вниманием к инициативам в области общественного здравоохранения, которые делают противогриппозные препараты важнейшим компонентом лечения сезонных заболеваний.
Обзор рынка лекарств от гриппа в США
Рынок лекарств от гриппа в США получил наибольшую долю выручки в 56,2% в 2024 году в Северной Америке, что отражает развитую систему здравоохранения страны и проактивные стратегии общественного здравоохранения. США являются основным драйвером рынка гриппа из-за своего значительного бремени сезонного гриппа, надежных программ вакцинации и постоянных инвестиций в НИОКР для новых методов лечения и вакцин. Потребители все чаще отдают приоритет профилактическим мерам и легко получают лекарства от гриппа. Сильная интеграция рекомендаций общественного здравоохранения, широкая доступность как вакцин, так и противовирусных препаратов, а также значительная популяция пациентов способствуют устойчивому спросу.
Обзор европейского рынка лекарств от гриппа
Европейский рынок противогриппозных препаратов, как ожидается, будет расширяться со среднегодовым темпом роста 2,5% в течение прогнозируемого периода, в первую очередь за счет устоявшихся государственных инициатив по вакцинации и лечению гриппа, строгих рекомендаций общественного здравоохранения и высокой заболеваемости сезонным гриппом. Сосредоточение региона на профилактическом здравоохранении в сочетании с передовой инфраструктурой здравоохранения и повышением осведомленности об осложнениях гриппа способствуют принятию противогриппозных препаратов. Европейские страны испытывают постоянный спрос как на вакцины, так и на противовирусные терапии в ответ на ежегодные вспышки.
Обзор рынка лекарств от гриппа в Великобритании
Ожидается, что рынок лекарств от гриппа в Великобритании будет расти с заметным среднегодовым темпом роста в течение прогнозируемого периода, что обусловлено надежными государственными программами вакцинации, растущим вниманием к общественному здравоохранению и желанием усилить защиту от сезонного гриппа. Хорошо налаженная система здравоохранения Великобритании и настойчивые рекомендации по ежегодной вакцинации от гриппа способствуют высокому уровню использования как вакцин, так и противовирусных препаратов среди населения. Приверженность страны инициативам общественного здравоохранения дополнительно стимулирует рост рынка.
Обзор рынка лекарств от гриппа в Германии
Ожидается, что рынок противогриппозных препаратов в Германии будет расширяться со значительным среднегодовым темпом роста в течение прогнозируемого периода, что будет обусловлено повышением осведомленности о безопасности общественного здравоохранения, сильным акцентом на профилактическую медицину и спросом на технологически передовые решения. Хорошо развитая инфраструктура здравоохранения Германии в сочетании с ее акцентом на высокие показатели вакцинации и эффективное управление заболеваниями способствует принятию противогриппозных препаратов, особенно в общей практике и больничных условиях. Интеграция передовой диагностики и предпочтение доказательно обоснованным методам лечения соответствуют ожиданиям местных потребителей и врачей.
Обзор рынка лекарств от гриппа в Азиатско-Тихоокеанском регионе
Рынок противогриппозных препаратов в Азиатско-Тихоокеанском регионе, как ожидается, будет расти самыми быстрыми темпами среднегодового темпа роста в течение прогнозируемого периода, что обусловлено ростом урбанизации, ростом располагаемых доходов и технологическими достижениями в здравоохранении в таких странах, как Китай, Япония и Индия. Большое и растущее население региона крайне восприимчиво к вспышкам гриппа, что приводит к повышению осведомленности и спроса на эффективные методы лечения и вакцины. Кроме того, поскольку Азиатско-Тихоокеанский регион расширяет свою инфраструктуру здравоохранения, а правительственные инициативы способствуют более широкому доступу к основным лекарственным средствам, доступность противогриппозных препаратов расширяется для более широкой потребительской базы
Обзор рынка лекарств от гриппа в Японии
Японский рынок противогриппозных препаратов набирает обороты в отношении противовирусных препаратов из-за высокой заболеваемости сезонным гриппом в стране, быстрой урбанизации и высокого спроса на эффективные варианты лечения. Японский рынок уделяет большое внимание общественному здравоохранению и охвату вакцинацией, а принятие противогриппозных препаратов обусловлено растущей осведомленностью об осложнениях, особенно среди стареющего населения. Интеграция современных противовирусных препаратов и постоянные усилия в области НИОКР стимулируют рост, поскольку Япония уделяет первоочередное внимание снижению бремени гриппа.
Обзор рынка лекарств от гриппа в Индии
Рынок противогриппозных препаратов в Индии составил значительную долю рынка в Азиатско-Тихоокеанском регионе в 2024 году, что объясняется расширением среднего класса страны, быстрой урбанизацией и повышением доступа к здравоохранению. Индия является крупным рынком с высоким бременем инфекционных заболеваний, и противогриппозные препараты становятся все более важными как в программах общественного здравоохранения, так и в частном здравоохранении. Стремление к улучшению инфраструктуры здравоохранения и доступность как отечественных, так и импортных противогриппозных препаратов являются ключевыми факторами, стимулирующими рынок в Индии, с прогнозируемым среднегодовым темпом роста 2,6%.
Доля рынка лекарств от гриппа
Индустрия лекарств от гриппа в основном представлена хорошо зарекомендовавшими себя компаниями, среди которых:
- AbbVie Inc. (США)
- АстраЗенека (Великобритания)
- BioNTech SE (Германия)
- Компания Bristol-Myers Squibb (США)
- Cipla (Индия)
- COCRYSTAL PHARMA, INC. (США)
- CSL (Великобритания)
- Daiichi Sankyo Company, Limited (Япония)
- Dr. Reddy's Laboratories Ltd. (Индия)
- Ф. Хоффманн-Ла Рош АГ (Швейцария)
- Gilead Sciences, Inc. (США)
- GSK plc. (Великобритания)
- Johnson & Johnson Services, Inc. (США)
- Merck & Co., Inc. (США)
- Moderna, Inc. (США)
- Новартис АГ (Швейцария)
- Novavax (США)
- Осивакс (Франция)
- Санофи СА (Франция)
Последние события на мировом рынке лекарств от гриппа
- В сентябре 2024 года FluMist от AstraZeneca был одобрен для самостоятельного введения в США. Это знаменует собой значительный прогресс, поскольку это первая вакцина от гриппа, которая не требует введения поставщиком медицинских услуг, что повышает доступность и удобство для людей, желающих провести профилактику гриппа. FluMist был одобрен FDA США для самостоятельного введения взрослыми до 49 лет или для введения родителем/опекуном лицам в возрасте от 2 до 17 лет.
- В августе 2024 года ученые Гарвардской медицинской школы разработали назальный спрей под названием Pathogen Capture and Neutralizing Spray (PCANS), или Profi. Этот спрей, как утверждается, защищает от гриппа, простуды и COVID-19 с эффективностью более 99,99%, образуя в носовой полости барьер, который захватывает и нейтрализует вирусы и бактерии.
- В мае 2024 года компании Sanofi и Novavax объявили о совместном эксклюзивном лицензионном соглашении по совместной коммерциализации текущей автономной адъювантной вакцины Novavax от COVID-19 по всему миру и разработке новой комбинации вакцины грипп-COVID-19. Целью этого партнерства является ускорение разработки комбинированных вакцин, обеспечивающих повышенное удобство и защиту для пациентов.
- В апреле 2023 года компания Sinovac Biotech Co., Ltd. объявила о недавнем вводе в эксплуатацию нового завода по производству вакцины от гриппа в Пекине. Это расширение увеличивает глобальные производственные мощности для вакцин от гриппа, способствуя более широкой доступности и поставкам
- В марте 2023 года Консультативный комитет FDA по вакцинам и связанным с ними биологическим продуктам (VRBPAC) собрался, чтобы определить состав вакцины для сезона гриппа в США 2023-2024 годов. Этот ежегодный процесс имеет решающее значение для обеспечения эффективности вакцин против циркулирующих штаммов вирусов гриппа.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.