Global Electronic Clinical Outcome Assessment Ecoa Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
1.70 Billion
USD
5.52 Billion
2024
2032
USD
1.70 Billion
USD
5.52 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 5.52 Billion | |
|
|
|
|
Сегментация мирового рынка электронной оценки клинических результатов (eCOA) по типу (оценка результатов, сообщаемая врачом (Clinician Reporting Outcome Assessment, CLINRO), оценка результатов, сообщаемых пациентом (Patient Reporting Outcome Assessment, PRO), оценка результатов, сообщаемых наблюдателем (Observer Reporting Outcome Assessment, OBSRO) и оценка результатов эффективности (Perfo)), модальность (решения на основе сайта, веб-решения и портативные устройства), конечный пользователь (контрактные исследовательские организации (CRO), фармацевтические и биотехнологические компании, компании по производству медицинского оборудования, больницы/поставщики медицинских услуг, компании по оказанию консалтинговых услуг, академические и исследовательские институты и другие), способ доставки (облачный и размещенный в Интернете) — тенденции отрасли и прогноз до 2032 года
Размер рынка электронной оценки клинических результатов (eCOA)
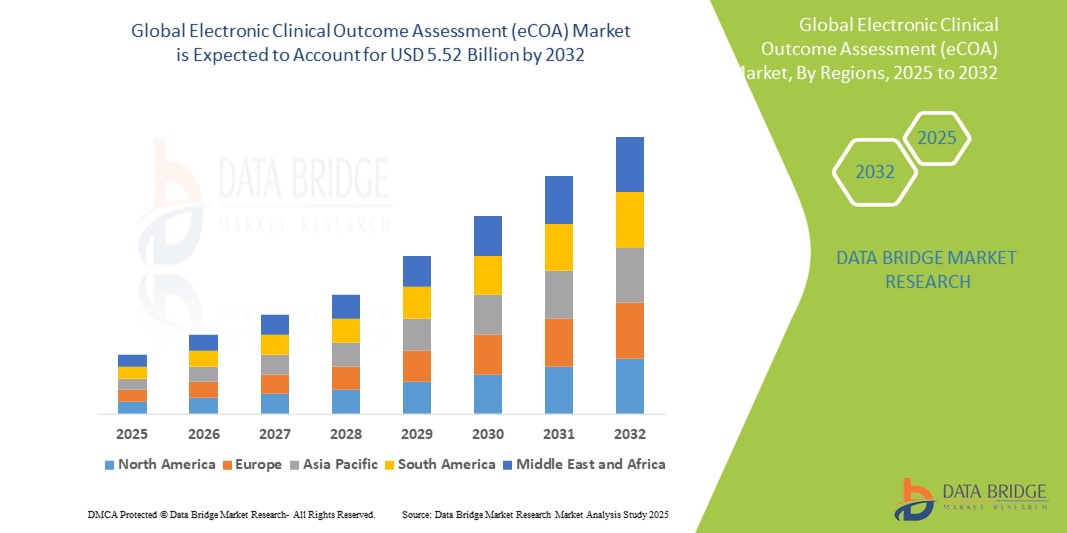
- Объем мирового рынка электронной оценки клинических результатов (eCOA) оценивался в 1,70 млрд долларов США в 2024 году и, как ожидается , достигнет 5,52 млрд долларов США к 2032 году при среднегодовом темпе роста 15,80% в течение прогнозируемого периода.
- Рост рынка обусловлен в первую очередь растущим внедрением цифровых технологий в клинические испытания и исследования в сфере здравоохранения, что способствует более точному и эффективному сбору и мониторингу данных о пациентах.
- Кроме того, растущий спрос на информацию о пациентах в режиме реального времени, более строгое соблюдение нормативных требований и повышенную целостность данных стимулирует внедрение решений eCOA в фармацевтических компаниях, контрактно-исследовательских организациях (CRO) и поставщиках медицинских услуг.
Анализ рынка электронной оценки клинических результатов (eCOA)
- Решения eCOA, позволяющие получать электронные данные о клинических результатах непосредственно от пациентов, лиц, осуществляющих уход, или врачей, становятся все более важными компонентами современных клинических испытаний и исследований в области здравоохранения благодаря повышенной точности данных, возможностям мониторинга в реальном времени и бесшовной интеграции с цифровыми экосистемами здравоохранения.
- Растущий спрос на eCOA обусловлен в первую очередь широким внедрением цифровых медицинских технологий, растущим акцентом на исследованиях, ориентированных на пациента, и растущим предпочтением удаленных, удобных для пользователя методов сбора данных, которые повышают эффективность исследований и соответствие требованиям.
- Северная Америка доминирует на рынке электронной оценки клинических результатов (eCOA) с самой большой долей выручки в 43,5% в 2024 году, что характеризуется ранним внедрением решений для цифровых клинических испытаний, сильными фармацевтическими и биотехнологическими секторами и нормативно-правовой базой, поддерживающей электронный сбор данных, при этом в США наблюдается существенный рост, обусловленный инновациями как от известных поставщиков, так и от новых поставщиков технологий, сосредоточенных на мобильных и облачных платформах.
- Ожидается, что Азиатско-Тихоокеанский регион станет самым быстрорастущим регионом на рынке электронной оценки клинических результатов (eCOA) в прогнозируемый период из-за увеличения активности клинических испытаний, увеличения инвестиций в здравоохранение и растущей осведомленности о преимуществах цифровых инструментов на развивающихся рынках, таких как Китай и Индия.
- Сегмент оценки результатов лечения, сообщаемых пациентами (PRO), доминирует на рынке электронной оценки клинических результатов (eCOA) с долей рынка 48,5% в 2024 году, что обусловлено его важнейшей ролью в получении мнений пациентов об эффективности лечения и качестве жизни, которые все чаще становятся приоритетными для спонсоров и регулирующих органов.
Область применения отчета и сегментация рынка электронной оценки клинических результатов (eCOA)
|
Атрибуты |
Электронная оценка клинических результатов (eCOA) Ключевые рыночные данные |
|
Охваченные сегменты |
|
|
Страны, охваченные |
Северная Америка
Европа
Азиатско-Тихоокеанский регион
Ближний Восток и Африка
Южная Америка
|
|
Ключевые игроки рынка |
|
|
Возможности рынка |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, анализ цен, анализ доли бренда, опрос потребителей, демографический анализ, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья/расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции рынка электронной оценки клинических результатов (eCOA)
«Повышение эффективности клинических испытаний с помощью ИИ и удаленного мониторинга пациентов»
- Значительной и ускоряющейся тенденцией на мировом рынке eCOA является углубление интеграции искусственного интеллекта (ИИ) и технологий удаленного мониторинга пациентов в платформы сбора данных клинических испытаний. Это слияние технологий значительно повышает точность, своевременность и пациентоориентированность оценок клинических результатов
- Например, ведущие поставщики eCOA, такие как Medidata и ERT, используют аналитику на основе искусственного интеллекта для выявления закономерностей в данных, сообщаемых пациентами, что позволяет на ранней стадии выявлять неблагоприятные события и улучшать процесс принятия решений по результатам испытаний. Аналогичным образом, носимые устройства в сочетании с платформами eCOA облегчают непрерывный мониторинг показателей здоровья пациентов в режиме реального времени за пределами традиционных визитов на места
- Интеграция ИИ в eCOA позволяет использовать такие функции, как прогнозная аналитика для соблюдения пациентом режима лечения, автоматизированные проверки качества данных и интеллектуальные оповещения о необычных реакциях пациента. Кроме того, возможности удаленного мониторинга предоставляют пациентам удобные, понятные интерфейсы для сообщения результатов из дома, что повышает полноту данных и вовлеченность
- Бесшовная интеграция систем eCOA с более широкими платформами цифрового здравоохранения и управления клиническими испытаниями позволяет спонсорам централизовать управление данными и оптимизировать рабочие процессы испытаний. С помощью унифицированных панелей мониторинга клинические группы могут отслеживать данные пациентов, производительность сайта и соответствие нормативным требованиям в режиме реального времени.
- Эта тенденция к более интеллектуальным, связанным и удобным для пациента решениям по клиническим результатам фундаментально меняет ожидания в отношении сбора данных клинических испытаний. Соответственно, такие компании, как Oracle Health и CRF Health, разрабатывают платформы eCOA с поддержкой ИИ с улучшенными возможностями прогнозирования и функциями удаленного сбора данных.
- Спрос на решения eCOA, включающие интеграцию искусственного интеллекта и удаленного мониторинга состояния пациентов, стремительно растет в фармацевтической, биотехнологической и медицинской отраслях, поскольку заинтересованные стороны все больше внимания уделяют эффективности испытаний, точности данных и опыту пациентов.
Динамика рынка электронной оценки клинических результатов (eCOA)
Водитель
«Растущий спрос на исследования, ориентированные на пациента, и точность цифровых данных»
- Растущее внимание к клиническим испытаниям, ориентированным на пациента, в сочетании с растущей потребностью в точном сборе цифровых данных в режиме реального времени является существенным фактором повышенного спроса на решения для электронной оценки клинических результатов (eCOA).
- Например, в январе 2024 года компания Medidata, входящая в Dassault Systèmes, представила новые усовершенствования на базе искусственного интеллекта для своей платформы eCOA, чтобы улучшить соблюдение пациентами режима лечения и качество данных в децентрализованных исследованиях. Ожидается, что такие инновации ключевых игроков отрасли будут способствовать росту рынка eCOA в прогнозируемый период
- Поскольку фармацевтические и биотехнологические компании стремятся оптимизировать процессы клинических испытаний и сократить время вывода продукции на рынок, платформы eCOA предлагают расширенные функции, такие как сбор данных в реальном времени, удаленная отчетность пациентов и автоматизированная проверка, что обеспечивает существенное улучшение по сравнению с традиционными методами на основе бумажных документов.
- Более того, растущее внедрение децентрализованных и гибридных моделей клинических испытаний позиционирует eCOA как важнейший компонент для удаленного сбора данных, улучшая взаимодействие с пациентами и поддерживая высокие стандарты соответствия нормативным требованиям.
- Способность платформ eCOA повышать эффективность испытаний посредством электронного сбора данных, многоязычной поддержки и интеграции с носимыми устройствами или мобильными приложениями является ключевым фактором, способствующим их внедрению в CRO, фармацевтических компаниях и научно-исследовательских институтах. Растущее внимание к сокращению показателей выбывания из клинических испытаний и улучшению целостности данных еще больше поддерживает широкомасштабную интеграцию решений eCOA в современные клинические исследования
Сдержанность/Вызов
«Опасения относительно конфиденциальности данных, соответствия нормативным требованиям и высоких затрат на внедрение»
- Проблемы, связанные с конфиденциальностью данных, соблюдением нормативных требований и высокими первоначальными затратами на внедрение платформ электронной оценки клинических результатов (eCOA), создают значительные трудности для более широкого внедрения на рынке.
- Поскольку системы eCOA подразумевают электронный сбор и передачу конфиденциальных данных о здоровье пациентов, они подпадают под строгие правила защиты данных, такие как HIPAA, GDPR и 21 CFR Часть 11, что делает соблюдение требований сложным и ресурсоемким для спонсоров и CRO.
- Например, несколько спонсоров клинических испытаний выразили осторожность в отношении полного перехода на системы eCOA из-за неопределенности в отношении правил локализации данных и сложности обеспечения соответствия требованиям трансграничных данных, особенно в многорегиональных испытаниях.
- Решение этих проблем требует надежной инфраструктуры безопасности данных, регулярных аудитов и соблюдения глобальных стандартов соответствия. Ведущие поставщики eCOA, такие как Oracle Health и Signant Health, вкладывают значительные средства в зашифрованные платформы и обучение нормативным требованиям для снижения риска и поддержания доверия со стороны заинтересованных сторон в ходе испытаний
- Кроме того, высокие первоначальные затраты, связанные с развертыванием систем eCOA, включая лицензионные сборы, закупку оборудования, обучение персонала и системную интеграцию, могут стать препятствием для входа, особенно для малых и средних исследовательских организаций. Хотя долгосрочные преимущества, такие как повышение точности данных и сокращение продолжительности испытаний, широко признаны, первоначальное финансовое бремя может ограничить принятие среди учреждений с ограниченными ресурсами
- Преодоление этих проблем с помощью масштабируемых моделей ценообразования, облачной доставки и постоянных инноваций в безопасных, удобных для пользователя платформах будет иметь решающее значение для более широкого и устойчивого внедрения решений eCOA в сфере клинических исследований.
Сфера применения электронной оценки клинических результатов (eCOA) на рынке
Рынок сегментирован по типу, модальности, конечному пользователю и способу доставки.
- По типу
На основе типа рынок электронной оценки клинических результатов (eCOA) сегментируется на результаты, сообщаемые пациентами (PRO), результаты, сообщаемые врачами (ClinRO), результаты, сообщаемые наблюдателями (ObsRO) и результаты эффективности (PerfO). Сегмент результатов, сообщаемых пациентами (PRO), занимал самую большую долю рынка в 48,5% в 2024 году, что обусловлено его ориентированным на пациента подходом к получению информации из первых рук об опыте, симптомах и результатах лечения пациентов. Инструменты PRO позволяют пациентам напрямую сообщать данные о своем здоровье в режиме реального времени через электронные платформы, повышая точность данных и вовлеченность пациентов, в конечном итоге улучшая качество клинических исследований.
Ожидается, что сегмент клинических результатов (ClinRO) значительно вырастет в течение прогнозируемого периода из-за растущей сложности клинических испытаний и потребности в точных и стандартизированных методах сбора данных. ClinRO включает оценки, проводимые обученными специалистами здравоохранения, предоставляя объективные и надежные данные для оценки клинических вмешательств, особенно в случаях, когда самоотчет пациента невозможен.
- По модальности
На основе модальности рынок электронной оценки клинических результатов (eCOA) сегментируется на решения на основе сайта, веб-решения и портативные устройства. Сегмент веб-решений занимал самую большую долю рынка доходов в 2024 году, что объясняется его удобными интерфейсами, легкой доступностью и более низкими инвестиционными потребностями. Веб-решения хранят клиентские данные на облачных серверах, доступных через Интернет с базовым компьютерным оборудованием и подключением к Интернету, предлагая гибкость для настройки и позволяя адаптироваться к конкретным потребностям клиентов.
Ожидается, что сегмент портативных устройств будет демонстрировать значительный рост в течение прогнозируемого периода, обусловленный растущим внедрением мобильных технологий в клинических испытаниях. Портативные устройства облегчают сбор данных в реальном времени и повышают приверженность пациентов, что делает их привлекательным вариантом для децентрализованных и удаленных клинических исследований.
- Конечным пользователем
На основе конечного пользователя рынок электронной оценки клинических результатов (eCOA) сегментируется на фармацевтические и биотехнологические компании, контрактные исследовательские организации (CRO), компании по производству медицинских приборов, больницы/поставщики медицинских услуг, консалтинговые компании, академические и научно-исследовательские институты и т. д. Сегмент фармацевтических и биотехнологических компаний доминирует на рынке, на долю которого в 2024 году пришлось 50,66% доли рынка. Это доминирование обусловлено важнейшей ролью решений eCOA в оптимизации сбора и анализа данных в ходе процессов разработки лекарственных препаратов, обеспечении соответствия нормативным стандартам и повышении точности данных клинических испытаний.
Ожидается, что сегмент контрактных исследовательских организаций (CRO) продемонстрирует существенный рост в прогнозируемый период, обусловленный растущей тенденцией крупных биофармацевтических и медицинских компаний передавать управление клиническими исследованиями на аутсорсинг. CRO предлагают комплексные услуги, охватывающие разработку исследований, набор пациентов, сбор и анализ данных, что делает их неотъемлемыми игроками в ландшафте eCOA.
По способу доставки
На основе способа доставки рынок электронной оценки клинических результатов (eCOA) сегментируется на облачные и веб-решения. Сегмент веб-решений занимал наибольшую долю рынка в 58,9% в 2025 году из-за его экономической эффективности по сравнению с облачными решениями. Веб-платформы предполагают более низкие начальные инвестиции в инфраструктуру для конечных пользователей, что снижает капитальные затраты для фармацевтических компаний, CRO и поставщиков медицинских услуг
Ожидается, что сегмент облачных решений продемонстрирует значительный рост в течение прогнозируемого периода, обусловленный его масштабируемостью, гибкостью и экономической эффективностью. Облачные платформы облегчают и ускоряют доступ к данным для заинтересованных сторон клинических испытаний, независимо от их местонахождения, что имеет решающее значение для многоцентровых испытаний.
Региональный анализ рынка электронной оценки клинических результатов (eCOA)
- Северная Америка доминирует на рынке электронной оценки клинических результатов (eCOA) с самой большой долей выручки в 43,5% в 2024 году, что обусловлено ранним внедрением решений для цифровых клинических испытаний, сильными фармацевтическими и биотехнологическими секторами, а также нормативно-правовой базой, поддерживающей электронный сбор данных.
- Регион пользуется преимуществами надежной нормативно-правовой базы, которая поддерживает цифровую трансформацию клинических исследований, побуждая фармацевтические компании и организации по контрактным исследованиям внедрять платформы eCOA для повышения точности данных и соблюдения нормативных требований.
- Кроме того, значительные инвестиции в НИОКР, хорошо налаженная инфраструктура здравоохранения и раннее внедрение децентрализованных и ориентированных на пациента клинических испытаний вносят значительный вклад в рост рынка. Присутствие ведущих поставщиков решений eCOA и CRO еще больше ускоряет региональное расширение электронных инструментов оценки клинических результатов
Анализ рынка электронной оценки клинических результатов (eCOA) в США
Рынок электронной оценки клинических результатов (eCOA) США получил наибольшую долю дохода в 79,6% в 2024 году в Северной Америке, что обусловлено лидерством страны в клинических испытаниях и быстрой цифровизацией практик клинических исследований. Регулирующие органы, такие как FDA, решительно выступают за использование цифровых инструментов для улучшения качества данных и вовлеченности пациентов, способствуя широкому внедрению систем eCOA. Кроме того, растущая потребность в децентрализованных и гибридных моделях клинических испытаний подпитывает спрос на удаленные платформы сбора данных пациентов в режиме реального времени. Рынок США также выигрывает от сильного финансирования НИОКР, большого присутствия фармацевтических гигантов и сложной ИТ-инфраструктуры здравоохранения.
Обзор европейского рынка электронной оценки клинических результатов (eCOA)
Европейский рынок электронной оценки клинических результатов (eCOA) по прогнозам будет расширяться со значительным среднегодовым темпом роста в течение прогнозируемого периода, подпитываемый растущим акцентом регулирования на реальных доказательствах, ориентированности на пациента и стандартизации данных в клинических испытаниях. Возросшая потребность в многоязычных и культурно адаптированных цифровых решениях в ЕС ускоряет принятие гибких масштабируемых платформ eCOA. Кроме того, рост академического исследовательского сотрудничества в сочетании с благоприятной политикой цифровой трансформации здравоохранения поддерживает региональный рост. Такие страны, как Германия, Великобритания и Франция, лидируют в принятии технологий в своих соответствующих экосистемах испытаний
Обзор рынка электронной оценки клинических результатов (eCOA) в Великобритании
Ожидается, что рынок электронной оценки клинических результатов (eCOA) в Великобритании будет расти с заметным среднегодовым темпом роста в течение прогнозируемого периода, подкрепленный сильным сектором биофармацевтических исследований и разработок и проактивными стратегиями цифрового здравоохранения NHS. Растущее число децентрализованных испытаний и нормативная ясность в отношении электронного сбора данных способствуют принятию рынка. Благодаря передовому ландшафту клинических исследований и значительным инвестициям в информатику здравоохранения в Великобритании наблюдается быстрое внедрение технологий eCOA для обеспечения соответствия, улучшения вовлеченности пациентов и эффективного отслеживания результатов.
Анализ рынка электронной оценки клинических результатов (eCOA) в Германии
Ожидается, что рынок электронной оценки клинических результатов (eCOA) в Германии будет расширяться со значительным среднегодовым темпом роста в течение прогнозируемого периода, что обусловлено репутацией страны в области клинических испытаний высочайшего качества и строгими законами о защите данных. Немецкие регулирующие органы подчеркивают надежность и безопасность клинических данных, побуждая спонсоров и CRO инвестировать в безопасные, проверенные решения eCOA. Кроме того, растущий спрос Германии на сбор данных в режиме реального времени в испытаниях фазы I-IV и ее мощная ИТ-инфраструктура здравоохранения способствуют дальнейшей интеграции технологий eCOA в исследования медицинских устройств и фармацевтики.
Обзор рынка электронной оценки клинических результатов (eCOA) в Азиатско-Тихоокеанском регионе
Рынок электронной оценки клинических результатов (eCOA) в Азиатско-Тихоокеанском регионе, как ожидается, будет расти самыми быстрыми темпами среднегодового темпа роста в прогнозируемый период с 2025 по 2032 год, что обусловлено ростом активности клинических исследований и цифровой трансформацией здравоохранения в таких странах, как Китай, Индия, Южная Корея и Япония. Расширение многонациональных испытаний и доступность различных групп пациентов поддерживают региональный рост. Государственные стимулы для внедрения цифровых медицинских платформ и растущий спрос на мобильные решения делают внедрение eCOA более осуществимым и широко распространенным в городских и полугородских регионах. Локальные партнерства между CRO и глобальными фармацевтическими компаниями еще больше продвигают внедрение eCOA.
Обзор рынка электронной оценки клинических результатов (eCOA) в Японии
Рынок электронной оценки клинических результатов (eCOA) в Японии набирает обороты из-за технологической сложности страны, старения населения и акцента на качественных данных в клинических испытаниях. Регулирующий орган Японии, PMDA, все более восприимчив к цифровым конечным точкам и инструментам удаленного сбора данных. Рынок также формируется за счет увеличения количества домашних и амбулаторных клинических испытаний, что обуславливает потребность в точных и удобных для пациентов системах eCOA. Ожидается, что интеграция с более широкими экосистемами eClinical и инструментами вовлечения пациентов на основе ИИ еще больше ускорит рост.
Обзор рынка электронной оценки клинических результатов (eCOA) в Индии
Рынок электронной оценки клинических результатов (eCOA) в Индии составил наибольшую долю выручки рынка в Азиатско-Тихоокеанском регионе в 2024 году, чему способствовал всплеск активности клинических испытаний, технически подкованное население и расширение возможностей фармацевтического производства. Эффективный с точки зрения затрат ландшафт CRO в Индии и поддерживающая государственная политика цифровизации здравоохранения побуждают глобальных спонсоров внедрять инструменты eCOA в отечественных испытаниях. Растущее проникновение смартфонов, рост телемедицины и улучшение интернет-подключения в городских и полугородских районах делают мобильные и облачные платформы eCOA более доступными и широко распространенными.
Электронная оценка клинических результатов (eCOA) Доля рынка
Индустрию электронной оценки клинических результатов (eCOA) в основном возглавляют хорошо зарекомендовавшие себя компании, в том числе:
- IQVIA (США)
- Кларио (США)
- Медидата (США)
- Veeva Systems (США)
- Технологии ресурсов Земли (США)
- Oracle Health Sciences (США)
- YPrime, LLC (США)
- ArisGlobal LLC (США)
- Castor EDC (Нидерланды)
- eClinicalWorks (США)
- Medio, Inc. (США)
- ClinOne (США)
- Signant Health (США)
- Clinical Ink, Inc. (США)
- Curebase, Inc. (США)
- Кайентис (Франция)
- Чашечка (Великобритания)
- Datacubed Health (США)
- HealthDiary, Inc. (США)
Последние разработки на мировом рынке электронной оценки клинических результатов (eCOA)
- В мае 2025 года Clario (США) приобрела бизнес eCOA компании WCG Clinical (США), что стало стратегическим шагом для укрепления ее лидерства в области цифровых решений для обработки конечных данных, в частности, для клинических испытаний в области нейробиологии. Это приобретение расширяет комплексную платформу конечных данных Clario, обеспечивая лучшую поддержку для сложных сред испытаний и еще больше укрепляя ее позиции в быстро меняющемся ландшафте eCOA
- В мае 2025 года Critical Path Institute (США) продолжил свою инициативу "eCOA: Getting Better Together", направленную на объединение спонсоров, поставщиков технологий и регулирующих органов. Эта совместная работа, которая продлится до марта 2025 года, направлена на установление лучших практик доконкурентного периода и общего лексикона для сбора данных eCOA, способствуя стандартизации и ускорению внедрения в различных регионах.
- В ноябре 2023 года Clinical Ink расширила свой пакет для взаимодействия с пациентами, включив в него инструмент поведенческой диагностики SPUR от Observia. Эта интеграция объединяет поведенческую оценку с модификацией образа жизни, eCOA, eSource и цифровыми биомаркерами, стремясь обеспечить более целостное понимание поведения пациентов и улучшить результаты испытаний.
- В октябре 2023 года Clario заключила стратегическое партнерство с Trial Data, поставщиком услуг децентрализованных клинических испытаний (DCT). Это сотрудничество укрепляет присутствие Clario на арене клинических испытаний в Китае, объединяя их опыт для предоставления современных решений для децентрализованных испытаний и продвижения подходов, ориентированных на пациента, в регионе.
- В декабре 2022 года Suvoda LLC, компания, занимающаяся технологиями клинических испытаний eCOA, представила свой набор инструментов для проектирования электронных оценок клинических результатов (eCOA). Этот набор инструментов создан для плавной интеграции с Suvoda IRT и eConsent, устраняя исторические недостатки в реализации eCOA и стремясь оптимизировать процесс проектирования.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.