Global Clinical Trial Management System Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
1.41 Billion
USD
3.87 Billion
2024
2032
USD
1.41 Billion
USD
3.87 Billion
2024
2032
| 2025 –2032 | |
| USD 1.41 Billion | |
| USD 3.87 Billion | |
|
|
|
|
Сегментация мирового рынка систем управления клиническими испытаниями (CTMS) по типу (корпоративные и локальные), доставке (веб-, облачные и локальные), компоненту (программное обеспечение и услуги), конечному пользователю (фармацевтические и биотехнологические компании, CRO и компании-производители медицинских приборов ) — тенденции отрасли и прогноз до 2032 г.
Размер рынка системы управления клиническими испытаниями (CTMS)
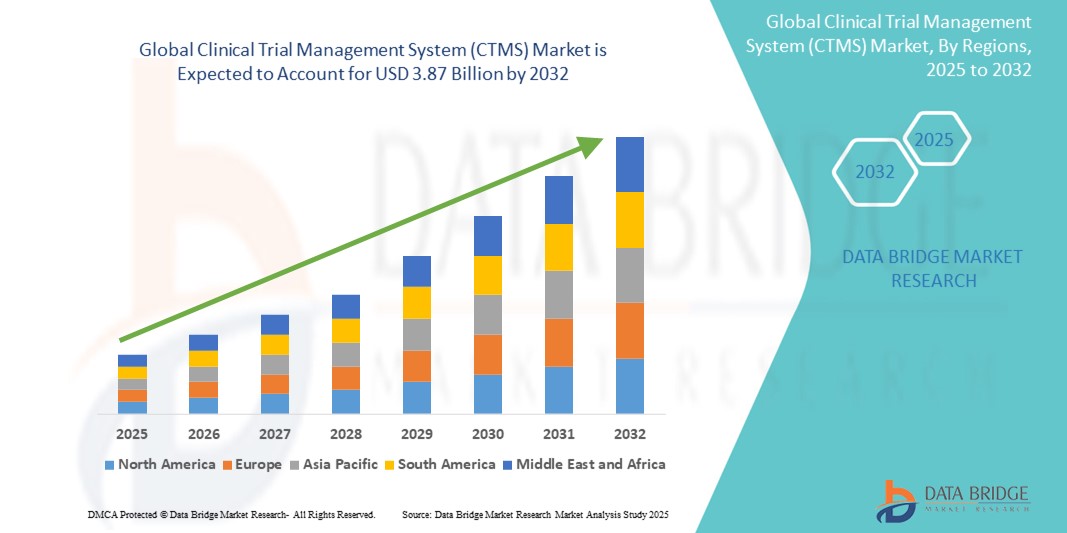
- Объем мирового рынка систем управления клиническими испытаниями (CTMS) оценивался в 1,41 млрд долларов США в 2024 году и, как ожидается , достигнет 3,87 млрд долларов США к 2032 году при среднегодовом темпе роста 13,4% в прогнозируемый период.
- Этот рост обусловлен такими факторами, как увеличение инвестиций в НИОКР, увеличение объема клинических испытаний и технологический прогресс.
Анализ рынка систем управления клиническими испытаниями (CTMS)
- Системы управления клиническими испытаниями (CTMS) являются важнейшими инструментами в фармацевтической и биотехнологической промышленности, предоставляя комплексные решения для планирования, отслеживания и управления клиническими испытаниями. Они играют важную роль в обеспечении соответствия нормативным требованиям, повышении точности данных и оптимизации операций испытаний
- Спрос на решения CTMS в значительной степени обусловлен растущим объемом клинических испытаний, ростом инвестиций в НИОКР и растущей сложностью клинических исследований. Интеграция передовых технологий, включая ИИ, машинное обучение и облачные вычисления, еще больше повышает эффективность и масштабируемость платформ CTMS
- Ожидается, что Северная Америка будет доминировать на рынке систем управления клиническими испытаниями (CTMS), занимая примерно 47,6% доли мирового рынка, что обусловлено присутствием крупных фармацевтических и биотехнологических компаний, сильной инфраструктурой клинических исследований и благоприятным государственным регулированием в поддержку клинических испытаний.
- Ожидается, что Азиатско-Тихоокеанский регион станет самым быстрорастущим регионом на рынке систем управления клиническими испытаниями (CTMS) со среднегодовым темпом роста 12,8%, что обусловлено ростом активности клинических испытаний, быстрым расширением инфраструктуры здравоохранения и ценовыми преимуществами при проведении клинических исследований в регионе.
- Ожидается, что сегмент на основе веб-технологий будет доминировать на рынке с наибольшей долей в 71,7%, что обусловлено его масштабируемостью, гибкостью и экономической эффективностью. Как предпочтительный выбор для управления клиническими испытаниями, облачные платформы CTMS обеспечивают доступ в режиме реального времени к данным испытаний, повышенную безопасность данных и снижение затрат на ИТ-инфраструктуру, поддерживая эффективное управление испытаниями
Область применения отчета и сегментация рынка системы управления клиническими испытаниями (CTMS)
|
Атрибуты |
Ключевые рыночные данные по системе управления клиническими испытаниями (CTMS) |
|
Охваченные сегменты |
|
|
Страны, охваченные |
Северная Америка
Европа
Азиатско-Тихоокеанский регион
Ближний Восток и Африка
Южная Америка
|
|
Ключевые игроки рынка |
|
|
Возможности рынка |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают анализ импорта и экспорта, обзор производственных мощностей, анализ потребления продукции, анализ ценовых тенденций, сценарий изменения климата, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья и расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции рынка систем управления клиническими испытаниями (CTMS)
«Интеграция ИИ и расширенной аналитики в CTMS»
- Одной из важных тенденций в развитии систем управления клиническими испытаниями является растущая интеграция искусственного интеллекта (ИИ), машинного обучения (МО) и расширенной аналитики.
- Эти технологии позволяют проводить анализ данных в режиме реального времени, получать прогнозные данные и автоматизировать процессы, повышая эффективность и точность управления клиническими испытаниями.
- Например , платформы CTMS на базе ИИ могут прогнозировать проблемы с регистрацией пациентов, оптимизировать выбор места и выявлять потенциальные задержки, значительно сокращая сроки и стоимость испытаний. Эта возможность особенно ценна в крупномасштабных многонациональных испытаниях, где быстрое принятие решений имеет решающее значение.
- Эти достижения трансформируют клинические испытания, улучшают результаты лечения пациентов и стимулируют спрос на платформы CTMS следующего поколения с передовой аналитикой данных и интеграцией искусственного интеллекта.
Динамика рынка систем управления клиническими испытаниями (CTMS)
Водитель
«Растущий спрос на эффективное управление клиническими испытаниями»
- Растущая сложность клинических испытаний, обусловленная развитием прецизионной медицины, персонализированной терапии и децентрализованных испытаний, в значительной степени обусловливает потребность в надежных системах управления клиническими испытаниями.
- Поскольку клинические испытания становятся все более глобальными и требуют все больше данных, потребность в доступе к данным в режиме реального времени, оптимизированных рабочих процессах и соблюдении нормативных требований становится критически важной для спонсоров, CRO и научно-исследовательских институтов.
- Расширенные платформы CTMS предлагают интегрированные решения для управления сайтом, регистрации пациентов, сбора данных и финансового отслеживания, повышая эффективность испытаний и сокращая эксплуатационные расходы.
Например,
- Согласно статье, опубликованной в журнале Journal of Clinical Research, в январе 2024 года ожидается значительный рост мирового рынка клинических испытаний, при этом к 2028 году более 30% испытаний будут проводиться по децентрализованным моделям. Этот сдвиг обуславливает спрос на передовые решения CTMS, которые могут обрабатывать сложные дизайны испытаний и удаленное управление данными.
- В результате ожидается, что потребность в эффективных системах управления клиническими испытаниями возрастет, что будет способствовать росту рынка CTMS.
Возможность
«Интеграция ИИ и расширенной аналитики в CTMS»
- Платформы CTMS на базе искусственного интеллекта могут повысить эффективность испытаний за счет автоматизации рутинных задач, улучшения набора пациентов и оптимизации анализа данных, что позволяет принимать более точные и быстрые решения.
- Эти платформы используют алгоритмы машинного обучения для прогнозирования задержек в судебных разбирательствах, оптимизации выбора места проведения и снижения показателей отсева, что значительно сокращает сроки и стоимость судебных разбирательств.
- Кроме того, системы CTMS на основе искусственного интеллекта могут предоставлять данные о пациентах в режиме реального времени, позволяя разрабатывать более персонализированные и адаптивные планы исследований.
Например,
- В марте 2025 года, согласно отчету Clinical Research News, несколько ведущих фармацевтических компаний интегрировали ИИ в свои платформы CTMS, что привело к ускорению набора пациентов на 40% и сокращению расходов на испытания на 30%. Ожидается, что эти достижения изменят управление клиническими испытаниями, стимулируя спрос на решения CTMS с поддержкой ИИ
- Интеграция ИИ в системы CTMS также может повысить точность данных, сократить эксплуатационные расходы и улучшить общие результаты испытаний, создавая значительные возможности для роста рынка.
Сдержанность/Вызов
«Высокие затраты на внедрение и проблемы безопасности данных»
- Высокая стоимость внедрения и обслуживания современных платформ CTMS может стать серьезным препятствием для малых и средних организаций клинических исследований (CRO) и биотехнологических компаний.
- Эти платформы часто требуют существенных первоначальных инвестиций, включая лицензирование программного обеспечения, модернизацию инфраструктуры и обучение персонала, что может быть непомерно дорогим для небольших организаций.
- Кроме того, опасения по поводу безопасности данных и соблюдения нормативных требований могут еще больше затруднить внедрение, поскольку клинические испытания генерируют конфиденциальные данные пациентов, которые необходимо защищать от утечки и несанкционированного доступа.
Например,
- В сентябре 2024 года в отчете журнала Data Protection Journal было отмечено растущее количество кибератак в секторе здравоохранения, причем данные клинических испытаний стали главной целью. Это побудило многие организации вкладывать значительные средства в кибербезопасность, что увеличило общую стоимость внедрения CTMS
- В результате высокая стоимость и проблемы безопасности данных, связанные с системами CTMS, могут ограничить их внедрение, особенно среди небольших организаций, замедляя общий рост рынка.
Масштаб рынка системы управления клиническими испытаниями (CTMS)
Рынок сегментирован по типу, поставке, компонентам и конечному пользователю.
|
Сегментация |
Субсегментация |
|
По типу |
|
|
Доставка |
|
|
По компонентам |
|
|
Конечным пользователем |
|
Ожидается, что в 2025 году на рынке будут доминировать веб-решения с наибольшей долей в сегменте доставки.
Ожидается, что сегмент веб-доставки будет доминировать на рынке систем управления клиническими испытаниями (CTMS) с наибольшей долей в 71,7% в 2025 году благодаря своей масштабируемости, гибкости и экономической эффективности. Как предпочтительный выбор для управления клиническими испытаниями, облачные платформы CTMS обеспечивают доступ к данным испытаний в режиме реального времени, повышенную безопасность данных и снижение затрат на ИТ-инфраструктуру, поддерживая эффективное управление испытаниями. Продолжающийся сдвиг в сторону децентрализованных испытаний и удаленного мониторинга пациентов еще больше способствует доминированию этого сегмента, стимулируя рост рынка.
Ожидается, что предприятие будет занимать наибольшую долю в прогнозируемом периоде на рынке.
Ожидается, что в 2025 году сегмент предприятий будет доминировать на рынке систем управления клиническими испытаниями (CTMS) с наибольшей долей рынка в 65,4% благодаря своей способности управлять сложными многоцентровыми клиническими испытаниями и поддерживать глобальные операции. Будучи ведущим выбором для крупномасштабных клинических исследований, платформы корпоративных CTMS предлагают доступ к данным в реальном времени, надежную аналитику и бесперебойное сотрудничество между заинтересованными сторонами клинических испытаний, повышая эффективность и результаты испытаний. Растущий спрос на интегрированные масштабируемые решения еще больше способствует его доминированию на рынке.
Региональный анализ рынка систем управления клиническими испытаниями (CTMS)
«Северная Америка занимает самую большую долю на рынке систем управления клиническими испытаниями (CTMS)»
- Ожидается, что Северная Америка будет доминировать на рынке систем управления клиническими испытаниями (CTMS), занимая 47,6% доли мирового рынка, что обусловлено присутствием крупных фармацевтических и биотехнологических компаний, сильной инфраструктурой клинических исследований и благоприятным государственным регулированием в поддержку клинических испытаний.
- США занимают значительную долю в 35,3% благодаря большому объему клинических испытаний, значительным инвестициям в НИОКР и раннему внедрению передовых технологий управления клиническими испытаниями.
- Устоявшаяся экосистема здравоохранения в регионе, обширная группа пациентов и ориентация на персонализированную медицину также способствуют его лидерству на рынке.
- Кроме того, наличие квалифицированных специалистов, развитой ИТ-инфраструктуры и надежной нормативно-правовой базы еще больше укрепляют рынок CTMS в Северной Америке.
«Прогнозируется, что в Азиатско-Тихоокеанском регионе будет зарегистрирован самый высокий среднегодовой темп роста на рынке систем управления клиническими испытаниями (CTMS)»
- Ожидается, что Азиатско-Тихоокеанский регион станет свидетелем самых высоких темпов роста рынка систем управления клиническими испытаниями (CTMS) с прогнозируемым среднегодовым темпом роста около 12,8%, что обусловлено ростом активности клинических испытаний, быстрым расширением инфраструктуры здравоохранения и ценовыми преимуществами при проведении клинических исследований в регионе.
- Такие страны, как Китай, Индия и Южная Корея, становятся ключевыми рынками благодаря развитию фармацевтической и биотехнологической отраслей, а также увеличению числа пациентов, участвующих в клинических испытаниях.
- Китай лидирует на региональном рынке благодаря значительной государственной поддержке клинических исследований, большим группам пациентов и расширяющимся возможностям производства биофармацевтической продукции.
- Прогнозируется, что в Индии будет зарегистрирован самый высокий среднегодовой темп роста на рынке CTMS, что обусловлено экономически эффективными клиническими испытаниями, растущим числом контрактных исследовательских организаций (CRO) и увеличением иностранных инвестиций в сектор здравоохранения.
Доля рынка системы управления клиническими испытаниями (CTMS)
Конкурентная среда рынка содержит сведения о конкурентах. Включены сведения о компании, ее финансах, полученном доходе, рыночном потенциале, инвестициях в исследования и разработки, новых рыночных инициативах, глобальном присутствии, производственных площадках и объектах, производственных мощностях, сильных и слабых сторонах компании, запуске продукта, широте и широте продукта, доминировании приложений. Приведенные выше данные касаются только фокуса компаний на рынке.
Основными лидерами рынка, работающими на рынке, являются:
- Адварра (США)
- ICON plc (Ирландия)
- Мератив (США)
- DSG, Inc. (США)
- ArisGlobal (США)
- Кларио (США)
- Оракул (США)
- Медидата (США)
- Фаунтейн (США)
- MedNet (США)
- IQVIA Inc. (США)
- SimpleTrials (США)
- Calyx Global Inc. (США)
- RealTime Software Solutions, LLC (США)
- LabCorp (США)
- Veeva Systems (США)
- Wipro (Индия)
- ФАРМАСИЛ (Великобритания)
Последние разработки на мировом рынке систем управления клиническими испытаниями (CTMS)
- В феврале 2025 года компания Medidata Solutions, ведущий поставщик технологий клинических испытаний, объявила о запуске своей новой системы управления клиническими испытаниями на базе искусственного интеллекта, разработанной для улучшения интеграции данных и аналитики в реальном времени. Система предлагает улучшенные возможности мониторинга испытаний, автоматизированную оценку рисков и оптимизированные рабочие процессы соответствия нормативным требованиям, направленные на ускорение сроков разработки лекарств и повышение эффективности испытаний.
- В ноябре 2024 года корпорация Oracle представила последнюю версию своей платформы CTMS с улучшенными облачными функциями, включая расширенные инструменты набора пациентов, поддержку удаленного мониторинга и интегрированные модули eConsent. Эти инновации предназначены для поддержки децентрализованных и гибридных клинических испытаний, улучшения вовлеченности участников и точности данных.
- В октябре 2024 года компания IQVIA Inc. представила свое решение CTMS следующего поколения, включающее алгоритмы машинного обучения для прогнозирования рисков испытаний и оптимизации распределения ресурсов. Платформа также предоставляет панели мониторинга в реальном времени и настраиваемые отчеты для улучшения принятия решений для организаций клинических исследований (CRO) и спонсоров
- В августе 2024 года Clario объявила о расширении своих предложений CTMS с интегрированными модулями электронного сбора данных (EDC) и результатов, сообщенных пациентами (PRO). Эта унифицированная система направлена на упрощение управления данными, повышение соответствия и улучшение дизайна испытаний, ориентированных на пациента. Обновленная платформа нацелена на повышение операционной эффективности и нормативной готовности
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.