Европейский рынок услуг по обеспечению безопасности сердца, по услугам (измерения ЭКГ/холтеровское мониторирование, измерения артериального давления , услуги по оценке безопасности сердца in vitro, визуализация сердечно-сосудистой системы, телеметрический мониторинг в реальном времени, централизованное перечитывание ЭКГ, неинвазивная визуализация сердца, физиологическое стресс-тестирование, тщательные исследования QT, моделирование TQT и реакции на воздействие, агрегация тромбоцитов и другие услуги), фаза (фаза 1, фаза 2 и фаза 3), тип (комплексные услуги и отдельные услуги), конечный пользователь ( фармацевтические и биофармацевтические компании, контрактные исследовательские организации и научно-исследовательские институты), отраслевые тенденции и прогноз до 2029 года.
Анализ рынка и идеи
Европейский рынок услуг по обеспечению безопасности сердца обусловлен такими факторами, как увеличение числа клинических испытаний, растущее число основных участников рынка и инновации в технологиях, которые повышают его спрос, а также увеличение инвестиций в исследования и разработки, что приводит к росту рынка. В настоящее время проводятся различные исследования, которые, как ожидается, создадут конкурентное преимущество для производителей в разработке новых и инновационных систем услуг по обеспечению безопасности сердца, что, как ожидается, предоставит различные другие возможности на рынке услуг по обеспечению безопасности сердца. Однако, строгие государственные правила утверждения, как ожидается, будут сдерживать рост.
Отчет о рынке услуг по обеспечению безопасности сердца в Европе содержит подробную информацию о доле рынка, новых разработках и анализе линейки продуктов, влиянии внутренних и локальных игроков рынка, анализирует возможности с точки зрения новых источников дохода, изменений в рыночных правилах, одобрений продуктов, стратегических решений, запусков продуктов, географических расширений и технологических инноваций на рынке. Чтобы понять анализ и рыночный сценарий, свяжитесь с нами для получения аналитического резюме, наша команда поможет вам создать решение по влиянию на доход для достижения желаемой цели. Масштабируемость и расширение бизнеса розничных подразделений в развивающихся странах различных регионов и партнерство с поставщиками для безопасного распространения машин и лекарственных препаратов являются основными факторами, которые стимулировали спрос на рынке в прогнозируемый период.
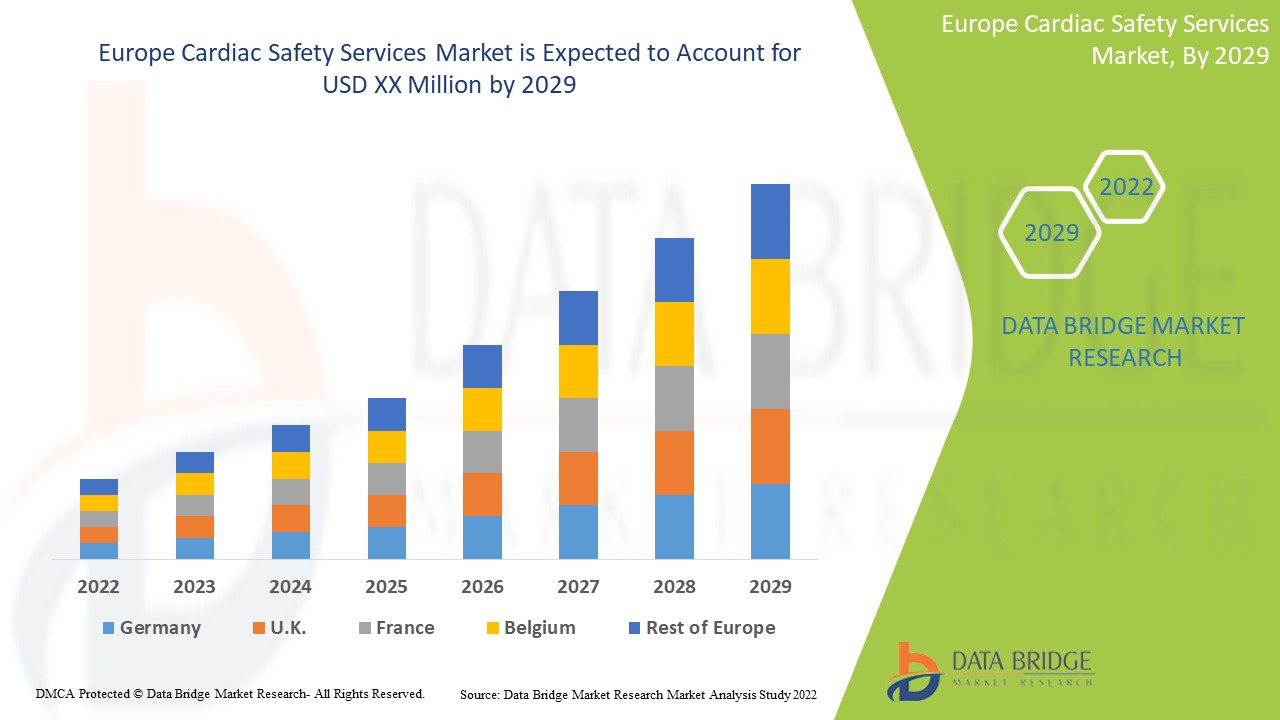
Европейский рынок услуг по обеспечению безопасности сердца является благоприятным и направлен на замедление прогрессирования заболевания. Data Bridge Market Research анализирует, что рынок услуг по обеспечению безопасности сердца в Европе будет расти в среднем на 14,8% в течение прогнозируемого периода с 2022 по 2029 год.
|
Отчет Метрика |
Подробности |
|
Прогнозируемый период |
2022-2029 |
|
Базовый год |
2021 |
|
Исторические годы |
2020 (Можно настроить на 2019 - 2014) |
|
Количественные единицы |
Доход в млн. долл. США, цены в долл. США |
|
Охваченные сегменты |
По услугам (измерения ЭКГ/холтеровское мониторирование, измерения артериального давления , услуги по оценке безопасности сердца in vitro, визуализация сердечно-сосудистой системы, телеметрический мониторинг в реальном времени, центральное перечитывание ЭКГ, неинвазивная визуализация сердца, физиологическое стресс-тестирование, тщательные исследования QT, моделирование TQT и реакции на воздействие, агрегация тромбоцитов и другие услуги), фаза (фаза 1, фаза 2 и фаза 3), тип (комплексные услуги и отдельные услуги), конечный пользователь ( фармацевтические и биофармацевтические компании, контрактные исследовательские организации и научно-исследовательские институты) |
|
Страны, охваченные |
Германия, Франция, Великобритания, Италия, Испания, Турция, Россия, Нидерланды, Швейцария, Бельгия и остальная Европа |
|
Охваченные участники рынка |
Koninklijke Philips NV, Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook и Celeron и другие. |
Определение рынка:
Услуги по обеспечению безопасности сердца обычно помогают поддерживать и разрабатывать клинические испытания и другие исследования, необходимые для мониторинга безопасности сердца. Спрос на рынок услуг по обеспечению безопасности сердца увеличился как в развитых, так и в развивающихся странах, и причиной этого является увеличение числа клинических испытаний и запусков продуктов. Рынок услуг по обеспечению безопасности сердца растет за счет внедрения инновационных продуктов, увеличения количества технологических продуктов и роста располагаемого дохода. Рынок будет расти в прогнозируемый период за счет исследования развивающихся рынков, стратегических инициатив участников рынка, увеличения расходов на здравоохранение.
Динамика рынка услуг по обеспечению безопасности сердца в Европе
Драйверы
- Увеличение количества клинических испытаний
Клиническое исследование — это хорошо структурированная система, которая существует уже сотни лет и по-прежнему является основой нормативных требований, необходимых для одобрения препарата. В последнее время в области клинических исследований наблюдается значительный прогресс, что увеличило количество клинических исследований и, как ожидается, будет способствовать росту рынка.
Произошли различные изменения в регулировании клинических испытаний, что привело к увеличению количества клинических испытаний и их положительных результатов.
Например,
- Согласно статье Medical News, значительно увеличилось количество испытаний из-за повышения качества клинических испытаний, например, обязательного обучения всего персонала. Кроме того, в 2017 году NIH заявил, что все исследователи и сотрудники должны пройти обучение по надлежащей клинической практике (GCP) в испытаниях, которые финансирует NIH
Увеличение расходов на здравоохранение и финансирование
Объем денежных средств, используемых страной на здравоохранение, и темпы их роста с течением времени зависят от широкого спектра экономических и социальных факторов, включая механизмы финансирования и структуру организации системы здравоохранения.
Расходы на здравоохранение увеличились в развитых странах и странах с развивающейся экономикой, поскольку располагаемый доход людей растет. Чем больше денег тратится на здравоохранение, тем здоровее население страны.
Возможность
- Рост разработки новых лекарств
Клинические испытания жизненно важны для открытия и разработки новых лекарств для лечения болезней. Это лучший способ для исследователей узнать, какие методы лечения работают или не работают на людях. Разработка лекарств характеризуется как формирование новых методов лечения в виде лекарств или устройств для лечения различных заболеваний, таких как рак, эндокринные, метаболические и другие.
- Таким образом, клинические испытания являются наиболее эффективным способом обеспечения безопасности и эффективности терапевтического препарата перед его выпуском на рынок и потреблением человеком, включая оценку безопасности для сердца, которая является неотъемлемой частью перед тем, как любой медицинский продукт поступит на рынок.
Сдержанность/Вызов
Правильная оценка и отчетность по клиническим данным по безопасности для сердца имеет важное значение. Одобрение и отзыв продукции для любых медицинских продуктов зависят от оценки безопасности для сердца. Поэтому необходимо предоставлять и проводить оценку безопасности для сердца в соответствии с правовой процедурой, в противном случае это приведет к позднему одобрению продукта, что, как ожидается, сдержит рост рынка.
Например,
- Согласно статье IQVIA, было 47 случаев отзыва лекарств после выхода на рынок в период с 1957 по 2007 год, в которых 45% из них были вызваны опасениями относительно сердечно-сосудистой токсичности. Аналогично, 27% потенциальных новых молекул лекарств, которые потерпели неудачу на доклинической стадии за последние два десятилетия, сделали это из-за сердечно-сосудистой токсичности, поскольку они не соответствовали требуемым нормативным требованиям
Сегментация рынка услуг по обеспечению безопасности сердца в Европе
Европейский рынок услуг по обеспечению безопасности сердца подразделяется на тип, услуги, фазу и конечного пользователя. Рост среди сегментов помогает вам анализировать нишевые карманы роста и стратегии подхода к рынку и определять основные области применения и разницу в ваших целевых рынках.
Услуги
- ЭКГ/холтеровские измерения
- Измерение артериального давления
- Услуги по оценке безопасности сердца in vitro
- Сердечно-сосудистая визуализация
- Телеметрический мониторинг в реальном времени
- Центральное перечитывание ЭКГ
- Неинвазивная кардиологическая визуализация
- Физиологическое стресс-тестирование
- Тщательные исследования QT
- TQT и моделирование реакции на воздействие
- Агрегация тромбоцитов
- Другие услуги
На основе услуг рынок услуг по обеспечению безопасности сердца сегментирован на следующие услуги: измерения ЭКГ /холтеровское мониторирование, измерения артериального давления, услуги по оценке безопасности сердца in vitro, визуализация сердечно-сосудистой системы , телеметрический мониторинг в реальном времени, централизованное перечитывание ЭКГ, неинвазивная визуализация сердца, физиологическое стресс-тестирование, тщательные исследования QT, моделирование TQT и реакции на воздействие, агрегация тромбоцитов и другие услуги.
Фаза
- Фаза 1
- Фаза 2
- Фаза 3
На основе фаз рынок услуг по обеспечению кардиобезопасности сегментируется на фазу 1, фазу 2 и фазу 3.
Тип
- Интегрированные услуги
- Отдельные услуги
По типу рынок услуг по обеспечению кардиобезопасности сегментируется на интегрированные услуги и автономные услуги.
Конечный пользователь
- Фармацевтические и биофармацевтические компании
- Контрактные исследовательские организации
- Научно-исследовательский институт
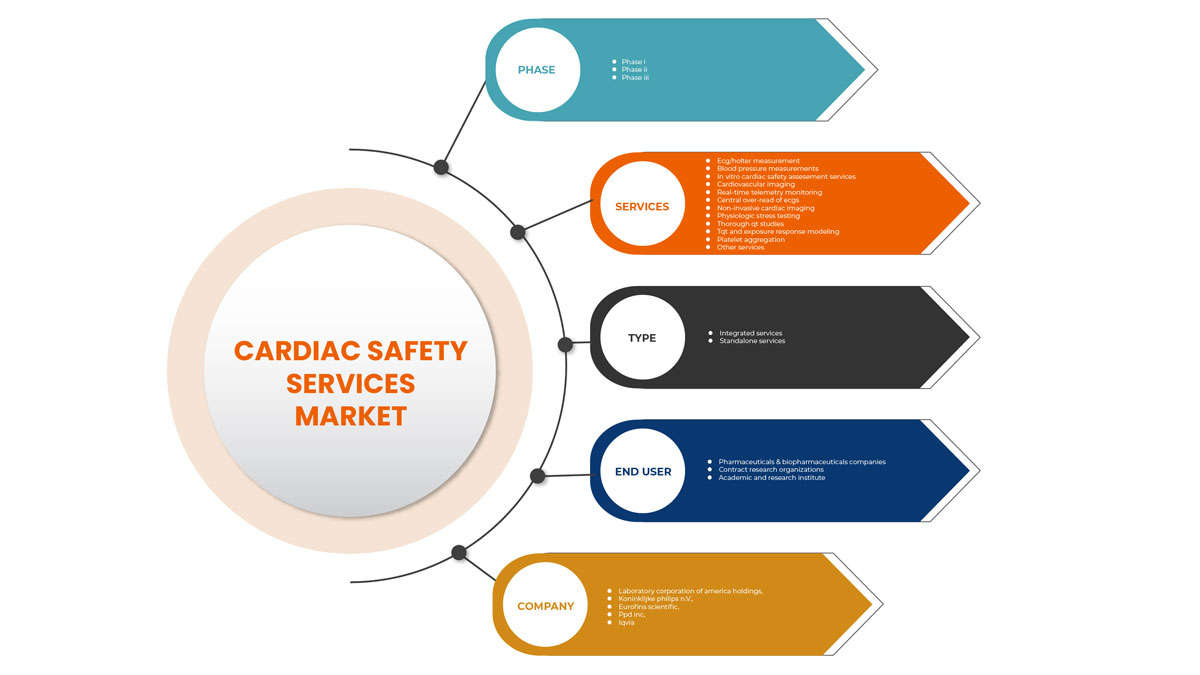
По признаку конечного пользователя рынок услуг по обеспечению безопасности сердца сегментирован фармацевтическими и биофармацевтическими компаниями, контрактными исследовательскими организациями, а также академическими и научно-исследовательскими институтами.
Региональный анализ/информация служб кардиологической безопасности
Проведен анализ услуг по обеспечению безопасности сердечно-сосудистых заболеваний, а также предоставлены сведения о размерах рынка и тенденциях по типу, услугам, фазе и конечному пользователю, как указано выше.
В отчете о службах безопасности кардиологических заболеваний рассматриваются следующие страны: Германия, Франция, Великобритания, Италия, Испания, Турция, Россия, Нидерланды, Швейцария, Бельгия и остальные страны Европы.
Ожидается, что Германия будет доминировать за счет роста разработки новых лекарственных препаратов и расходов на здравоохранение.
Раздел отчета по странам также содержит отдельные факторы, влияющие на рынок, и изменения в регулировании рынка, которые влияют на текущие и будущие тенденции рынка. Такие данные, как анализ цепочки создания стоимости сверху и снизу, технические тенденции и анализ пяти сил Портера, тематические исследования, являются некоторыми из указателей, используемых для прогнозирования рыночного сценария для отдельных стран. Кроме того, при предоставлении прогнозного анализа данных по странам учитываются наличие и доступность европейских брендов и их проблемы из-за большой или малой конкуренции со стороны местных и отечественных брендов, влияние внутренних тарифов и торговых путей.
Анализ конкурентной среды и услуг по обеспечению безопасности сердечно-сосудистых заболеваний
Конкурентная среда европейского рынка услуг по обеспечению безопасности сердца содержит сведения о конкурентах. Включены сведения о компании, ее финансах, полученном доходе, рыночном потенциале, инвестициях в исследования и разработки, новых рыночных инициативах, присутствии в Европе, производственных площадках и объектах, производственных мощностях, сильных и слабых сторонах компании, запуске продукта, широте и широте продукта и доминировании в применении. Приведенные выше данные касаются только фокуса компаний на рынке услуг по обеспечению безопасности сердца.
Основными игроками на рынке являются Koninklijke Philips NV, Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook и Celerion и другие.
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Рыночные данные анализируются и оцениваются с использованием рыночных статистических и когерентных моделей. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в рыночном отчете. Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Помимо этого, модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, анализ доли рынка компании, стандарты измерения, анализ Европы против региона и доли поставщика. Пожалуйста, запросите звонок аналитика в случае дальнейшего запроса.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Содержание
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE CARDIAC SAFETY SERVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTERS FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6 INDUSTRY INSIGHT
6.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.2 PATENT ANALYSIS
6.3 PATENT FLOW DIAGRAM
6.4 KEY PATIENT ENROLLMENT STRATEGIES
6.5 PRICING STRATEGY
7 EUROPE CARDIAC SAFETY SERVICES MARKET: REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASE IN THE NUMBER OF CLINICAL TRIALS
8.1.2 INCREASE IN HEALTHCARE EXPENDITURE AND FUNDING
8.1.3 INCREASE IN STRATEGIC INITIATIVES BY MAJOR MARKET PLAYERS
8.1.4 INCREASE IN R&D ACTIVITIES
8.2 RESTRAINTS
8.2.1 HIGH COST OF CARDIAC SAFETY EVALUATION
8.2.2 STRICT REGULATORY
8.3 OPPORTUNITIES:
8.3.1 INCREASE IN NEW DRUG DEVELOPMENT
8.3.2 RISE IN THE EXPANSION OF THE CARDIAC SAFETY SERVICES
8.4 CHALLENGES
8.4.1 TIME-CONSUMING PROCEDURE
8.4.2 LACK OF SKILLED PERSON TO OPERATE DEVICES DURING CARDIAC SAFETY EVALUATION
9 EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES
9.1 OVERVIEW
9.2 ECG/HOLTER MEASUREMENTS
9.3 BLOOD PRESSURE MEASUREMENTS
9.4 IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES
9.4.1 HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS
9.4.1.1 1 CONCENTRATIONS
9.4.1.2 4 CONCENTRATIONS
9.4.2 COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA)
9.4.2.1 3 CONCENTRATIONS
9.4.2.2 5 CONCENTRATIONS
9.4.3 IN VITRO HERG ASSAY
9.4.4 OTHERS
9.5 CARDIOVASCULAR IMAGING
9.6 REAL TIME TELEMETRY MONITORING
9.7 CENTRAL OVER-READ OF ECGS
9.8 NON-INVASIVE CARDIAC IMAGING
9.9 PHYSIOLOGIC STRESS TESTING
9.1 THOROUGH QT STUDIES
9.11 TQT AND EXPOSURE RESPONSE MODELLING
9.12 PLATELET AGGREGATION
9.13 OTHERS
10 EUROPE CARDIAC SAFETY SERVICES MARKET, BY PHASE
10.1 OVERVIEW
10.2 PHASE I
10.3 PHASE II
10.4 PHASE III
11 EUROPE CARDIAC SAFETY SERVICES MARKET, BY TYPE
11.1 OVERVIEW
11.2 INTEGRATED SERVICES
11.3 STANDALONE SERVICES
12 EUROPE CARDIAC SAFETY SERVICES MARKET, BY END USER
12.1 OVERVIEW
12.2 PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES
12.3 CONTRACT RESEARCH ORGANIZATIONS
12.4 ACADEMIC AND RESEARCH INSTITUTE
13 EUROPE CARDIAC SAFETY SERVICES MARKET, BY REGION
13.1 EUROPE
13.1.1 GERMANY
13.1.2 FRANCE
13.1.3 U.K.
13.1.4 ITALY
13.1.5 SPAIN
13.1.6 TURKEY
13.1.7 RUSSIA
13.1.8 NETHERLANDS
13.1.9 SWITZERLAND
13.1.10 BELGIUM
13.1.11 REST OF EUROPE
14 EUROPE CARDIAC SAFETY SERVICES MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 COMPANY PROFILE
15.1 EUROFINS SCIENTIFIC
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.1.5.1 AGREEMENTS
15.1.5.2 ACQUISITION
15.2 PPD INC. (SUBSIDIARY OF THERMO FISHER SCIENTIFIC INC)
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.2.5.1 INVESTMENT
15.3 KONINKLIJKE PHILIPS N.V.
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.3.5.1 ACQUISITION
15.4 IQVIA
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.4.5.1 ACQUISITION
15.5 LABORATORY CORPORATION OF AMERICA HOLDINGS
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.5.5.1 NEW LABORATORY
15.5.5.2 ACQUISITION
15.6 BANOOK
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.6.3.1 AGREEMENT
15.7 BIOTRIAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.7.3.1 NEW CENTER OPENING
15.8 CELERION
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.8.3.1 NEW CENTER OPENING
15.9 CERTARA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.9.4.1 CONTRACT
15.9.4.2 ACQUISITION
15.1 CLARIO
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.10.3.1 PRODUCT EXPANSION
15.11 MEDPACE
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.11.4.1 ACQUISITION
15.12 NCARDIA
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.12.3.1 PARTNERSHIP
15.13 NEXEL CO., LTD
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.13.3.1 JOINT VENTURE
15.13.3.2 PARTNERSHIP
15.14 PHYSIOSTIM
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.14.3.1 PARTNERSHIP
15.15 RICHMOND PHARMACOLOGY
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.15.3.1 EVENT
15.16 SGS SA
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.16.4.1 ACQUISITION
15.17 SHANGHAI MEDICILON INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENTS
15.17.3.1 PARTNERSHIP
15.17.3.2 PARTNERSHIP
16 QUESTIONNAIRE
17 RELATED REPORTS
Список таблиц
TABLE 1 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 2 PROBABILITY OF SUCCESS BY CLINICAL TRIAL PHASE TO THERAPEUTIC AREA
TABLE 3 MORTALITY RATES FROM CLINICAL TRIALS AND EUROPEAN SAFETY AND EXPOSURE SURVEY (ESES), DEATHS PER 100 (PYE)
TABLE 4 ADHERENCE RATE TO COMMON CARDIOVASCULAR MEDICATION
TABLE 5 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 6 INITIATIVES TO INCREASE ENROLLMENT IN CLINICAL TRIALS AMONG UNDERREPRESENTED POPULATIONS
TABLE 7 ESTIMATED COST OF CARDIAC SAFETY EVALUATION DEVICES
TABLE 8 EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 9 EUROPE ECG/HOLTER MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE BLOOD PRESSURE MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 13 EUROPE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 14 EUROPE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 15 EUROPE CARDIOVASCULAR IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 EUROPE REAL TIME TELEMETRY MONITORING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE CENTRAL OVER-READ OF ECGS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE NON-INVASIVE CARDIAC IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE PHYSIOLOGIC STRESS TESTING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE THOROUGH QT STUDIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE TQT AND EXPOSURE RESPONSE MODELLING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE PLATELET AGGREGATION IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE OTHERS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 25 EUROPE PHASE I IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE PHASE II IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE PHASE III IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 29 EUROPE INTEGRATED SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE STANDALONE SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 EUROPE PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE CONTRACT RESEARCH ORGANIZATIONS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE ACADEMIC AND RESEARCH INSTITUTE IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 36 EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 37 EUROPE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 38 EUROPE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 39 EUROPE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 40 EUROPE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 41 EUROPE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 42 EUROPE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 43 GERMANY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 44 GERMANY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 45 GERMANY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 46 GERMANY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 47 GERMANY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 48 GERMANY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 GERMANY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 50 FRANCE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 51 FRANCE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 52 FRANCE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 53 FRANCE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 54 FRANCE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 55 FRANCE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 FRANCE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 57 U.K. CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 58 U.K. IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 59 U.K. HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 60 U.K. COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 61 U.K. CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 62 U.K. CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 63 U.K. CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 64 ITALY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 65 ITALY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 66 ITALY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 67 ITALY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 68 ITALY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 69 ITALY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 70 ITALY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 SPAIN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 72 SPAIN IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 73 SPAIN HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 74 SPAIN COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 75 SPAIN CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 76 SPAIN CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 77 SPAIN CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 78 TURKEY CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 79 TURKEY IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 80 TURKEY HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 81 TURKEY COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 82 TURKEY CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 83 TURKEY CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 84 TURKEY CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 85 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 86 RUSSIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 87 RUSSIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 88 RUSSIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 89 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 90 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 91 RUSSIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 92 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 93 NETHERLANDS IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 94 NETHERLANDS HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 95 NETHERLANDS COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 96 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 97 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 98 NETHERLANDS CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 99 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 100 SWITZERLAND IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 101 SWITZERLAND HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 102 SWITZERLAND COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 103 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 104 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 105 SWITZERLAND CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 106 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 107 BELGIUM IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 108 BELGIUM HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 109 BELGIUM COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 110 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 111 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 112 BELGIUM CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 113 REST OF EUROPE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
Список рисунков
FIGURE 1 EUROPE CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE CARDIAC SAFETY SERVICES MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE CARDIAC SAFETY SERVICES MARKET: DROC ANALYSIS
FIGURE 4 EUROPE CARDIAC SAFETY SERVICES MARKET: EUROPE VS COUNTRY MARKET ANALYSIS
FIGURE 5 EUROPE CARDIAC SAFETY SERVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE CARDIAC SAFETY SERVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE CARDIAC SAFETY SERVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE CARDIAC SAFETY SERVICES MARKET: MARKET END USER GRID
FIGURE 9 EUROPE CARDIAC SAFETY SERVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN DEMAND FOR CARDIAC SAFETY SERVICES ARE EXPECTED TO DRIVE THE EUROPE CARDIAC SAFETY SERVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 ECG/HOLTER MEASUREMENTS SUBSTITUTE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE CARDIAC SAFETY SERVICES MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM FOR ANY RANDOM DRUG
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE CARDIAC SAFETY SERVICES MARKET
FIGURE 15 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2021
FIGURE 16 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2022-2029 (USD MILLION)
FIGURE 17 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES, CAGR (2022-2029)
FIGURE 18 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES, LIFELINE CURVE
FIGURE 19 EUROPE CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2021
FIGURE 20 EUROPE CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2022-2029 (USD MILLION)
FIGURE 21 EUROPE CARDIAC SAFETY SERVICES MARKET: BY PHASE, CAGR (2022-2029)
FIGURE 22 EUROPE CARDIAC SAFETY SERVICES MARKET: BY PHASE, LIFELINE CURVE
FIGURE 23 EUROPE CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2021
FIGURE 24 EUROPE CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 25 EUROPE CARDIAC SAFETY SERVICES MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 26 EUROPE CARDIAC SAFETY SERVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 27 EUROPE CARDIAC SAFETY SERVICES MARKET: BY END USER, 2021
FIGURE 28 EUROPE CARDIAC SAFETY SERVICES MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 29 EUROPE CARDIAC SAFETY SERVICES MARKET: BY END USER, CAGR (2022-2029)
FIGURE 30 EUROPE CARDIAC SAFETY SERVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 32 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 33 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 34 EUROPE CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 35 EUROPE CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 36 EUROPE CARDIAC SAFETY SERVICES MARKET: COMPANY SHARE 2021 (%)

Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.