Europe Anti Nuclear Antibody Test Market
Размер рынка в млрд долларов США
CAGR :
% 
 USD
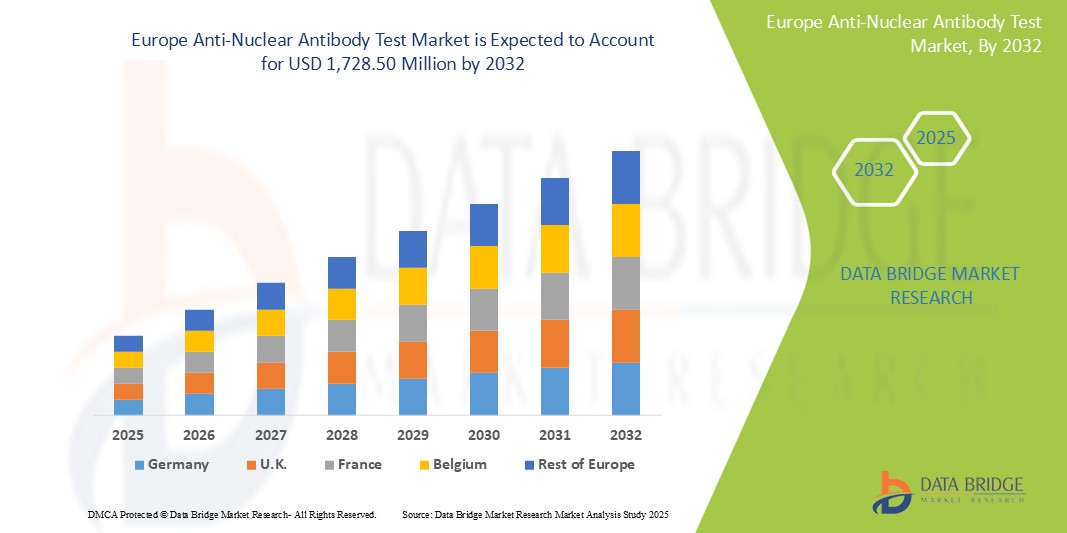
659.47 Million
USD
1,728.50 Million
2024
2032
USD
659.47 Million
USD
1,728.50 Million
2024
2032
| 2025 –2032 | |
| USD 659.47 Million | |
| USD 1,728.50 Million | |
|
|
|
|
Сегментация рынка тестов на антиядерные антитела в Европе по типу антител (извлекаемые ядерные антигены (ENA), анти-DSDNA и гистоны, антитела анти-DFS70, анти-PM-SCL, антитела антицентромеры, анти-SP100 и другие), продукту (инструменты, расходные материалы и реагенты, а также услуги), методу (ИФА, непрямая иммунофлуоресценция (НИФ), блоттинг-тест, антигенный микрочип, методы на основе геля, мультиплексный анализ, проточная цитометрия, пассивная гемагглютинация (ПГА) и другие), применению (аутоиммунные заболевания и инфекционные заболевания), по конечному пользователю (больницы, лаборатории, диагностические центры, научно-исследовательские институты и другие), каналу сбыта (прямые торги, розничные продажи и сторонние дистрибьюторы и другие) - отраслевые тенденции и прогноз до 2032 г.
Объем рынка тестов на антинуклеарные антитела в Европе
- Объем европейского рынка тестов на антинуклеарные антитела в 2024 году оценивался в 659,47 млн долларов США , а к 2032 году, как ожидается, достигнет 1 728,50 млн долларов США , при среднегодовом темпе роста 12,8% в прогнозируемый период.
- Этот рост обусловлен такими факторами, как рост распространенности аутоиммунных заболеваний, технологический прогресс в методах диагностики, а также повышение осведомленности общественности и инициативы по раннему выявлению заболеваний.
Анализ рынка тестов на антинуклеарные антитела в Европе
- Тесты на антинуклеарные антитела (ANA) являются важнейшими диагностическими инструментами, используемыми для обнаружения аутоантител в крови, помогая в диагностике аутоиммунных заболеваний , таких как системная красная волчанка (СКВ), ревматоидный артрит и синдром Шегрена. Эти тесты играют важную роль в раннем выявлении и лечении этих состояний
- Спрос на тесты на АНА в значительной степени обусловлен ростом распространенности аутоиммунных заболеваний, повышением осведомленности о ранней диагностике и достижениями в диагностических технологиях.
- Ожидается, что Германия будет доминировать на европейском рынке тестов на антинуклеарные антитела с долей рынка 25,6% благодаря своей развитой системе здравоохранения, высокой осведомленности пациентов и наличию передовых технологий тестирования.
- Италия, как ожидается, станет самой быстрорастущей страной Европы на рынке тестов на антинуклеарные антитела с CAGR 13,4%, что связано с ростом аутоиммунных заболеваний, таких как волчанка, ревматоидный артрит и синдром Шегрена. Этот рост заболеваемости привел к повышению спроса на раннее и точное диагностическое тестирование, включая тесты на ANA
- Непрямая иммунофлюоресценция (НИФ), как ожидается, будет доминировать на рынке с долей рынка 59,9%. Это доминирование объясняется его статусом золотого стандарта для тестирования ANA, предлагая высокую чувствительность и способность обнаруживать широкий спектр аутоантител.
Область применения отчета и сегментация европейского рынка тестов на антинуклеарные антитела
|
Атрибуты |
Основные сведения о рынке тестов на антиядерные антитела в Европе |
|
Охваченные сегменты |
|
|
Страны, охваченные |
Европа
|
|
Ключевые игроки рынка |
|
|
Возможности рынка |
|
|
Информационные наборы данных с добавленной стоимостью |
Помимо информации о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают анализ импорта и экспорта, обзор производственных мощностей, анализ потребления продукции, анализ ценовых тенденций, сценарий изменения климата, анализ цепочки поставок, анализ цепочки создания стоимости, обзор сырья и расходных материалов, критерии выбора поставщиков, анализ PESTLE, анализ Портера и нормативную базу. |
Тенденции европейского рынка тестов на антинуклеарные антитела
«Технологические достижения в тестировании на антинуклеарные антитела для диагностики аутоиммунных заболеваний»
- Одной из заметных тенденций на европейском рынке тестов на антинуклеарные антитела является растущая интеграция передовых диагностических технологий, включая автоматизированные системы и мультиплексные анализы, для повышения точности и эффективности.
- Эти инновации повышают точность диагностики, позволяя одновременно обнаруживать несколько аутоантител, сокращая время выполнения анализа и сводя к минимуму человеческие ошибки, тем самым способствуя ранней диагностике и персонализированному лечению.
- Например, современные платформы мультиплексного анализа позволяют обнаружить широкий спектр аутоантител в одном тесте, предоставляя комплексные профили пациентов, которые имеют решающее значение для лечения сложных аутоиммунных заболеваний, таких как системная красная волчанка (СКВ) и ревматоидный артрит.
- Эти достижения трансформируют ландшафт аутоиммунной диагностики, улучшают результаты лечения пациентов и стимулируют спрос на решения для тестирования ANA следующего поколения с повышенной чувствительностью и специфичностью.
Динамика рынка тестов на антиядерные антитела в Европе
Водитель
«Растущий уровень распространенности аутоиммунных заболеваний»
- Растущая заболеваемость аутоиммунными заболеваниями, такими как системная красная волчанка (СКВ), ревматоидный артрит, синдром Шегрена и системный склероз, значительно увеличивает спрос на тесты на антинуклеарные антитела.
- Аутоиммунные заболевания становятся все более распространенными из-за сочетания генетических, экологических и образ жизни факторов, что увеличивает потребность в точной и ранней диагностике для лечения этих сложных состояний.
- По мере роста осведомленности об аутоиммунных заболеваниях поставщики медицинских услуг все чаще используют тесты на АНА в качестве части плановой диагностики для улучшения результатов лечения пациентов и снижения долгосрочных расходов на здравоохранение.
Например,
- По данным отчета, опубликованного Европейским обществом ревматологов, в марте 2024 года распространенность СКВ в Европе оценивается примерно в 0,1–0,2% населения, причем значительной части этих пациентов требуется регулярное тестирование на АНА для лечения и мониторинга заболевания.
- Поскольку распространенность аутоиммунных заболеваний продолжает расти, ожидается, что спрос на тесты на АНА будет расти, что будет способствовать ранней диагностике, персонализированному лечению и улучшению результатов лечения пациентов.
Возможность
«Интеграция передовых диагностических технологий»
- Технологические достижения в области АНА-тестирования, включая мультиплексные анализы, автоматизированные платформы и диагностические инструменты на основе искусственного интеллекта (ИИ), открывают значительные возможности для роста на рынке.
- Эти технологии обеспечивают более быструю, точную и экономически эффективную диагностику за счет одновременного обнаружения нескольких аутоантител, сокращения времени выполнения анализа и минимизации человеческих ошибок.
- Кроме того, системы на базе искусственного интеллекта могут анализировать результаты тестов в режиме реального времени, предоставляя прогнозную информацию о развитии заболевания и помогая врачам принимать более обоснованные решения.
Например ,
- В январе 2025 года, согласно исследованию, опубликованному в журнале Journal of Autoimmunity, алгоритмы ИИ, разработанные для тестирования ANA, продемонстрировали более высокую точность в выявлении ранних стадий аутоиммунных заболеваний, снижение ложноположительных результатов и повышение общей эффективности диагностики. Такая интеграция может значительно улучшить результаты лечения пациентов за счет содействия раннему вмешательству и целенаправленной терапии.
- Ожидается, что внедрение этих передовых технологий будет способствовать росту рынка, поскольку лаборатории и поставщики медицинских услуг стремятся улучшить диагностические возможности и уход за пациентами.
Сдержанность/Вызов
«Высокая стоимость современных диагностических систем»
- Несмотря на преимущества, высокая стоимость современных систем тестирования ANA и мультиплексных платформ представляет собой существенное препятствие для роста рынка, особенно для небольших лабораторий и медицинских учреждений с ограниченным бюджетом.
- Первоначальные инвестиции, необходимые для автоматизированных систем и специализированного испытательного оборудования, могут оказаться непомерно высокими, что скажется на доступности и доступности расширенной диагностики.
- Это финансовое бремя может задержать внедрение передовых технологий, особенно в регионах с ограниченным бюджетом здравоохранения, что ограничивает доступ к передовой аутоиммунной диагностике.
Например,
- Согласно отчету Европейской ассоциации производителей диагностического оборудования, в ноябре 2024 года высокая стоимость платформ для мультиплексного анализа и автоматизированных систем тестирования ANA останется серьезной проблемой, поскольку небольшим лабораториям будет сложно оправдать инвестиции без существенного роста объемов или поддержки возмещения расходов.
- Следовательно, этот ценовой барьер может привести к различиям в качестве диагностики, ограничивая доступ к раннему и точному выявлению аутоиммунных заболеваний для многих пациентов.
Объем европейского рынка тестов на антиядерные антитела
Рынок сегментирован по типу антител, продукту, технологии, применению, конечному пользователю и каналу сбыта.
|
Сегментация |
Субсегментация |
|
По типу антител |
|
|
По продукту |
|
|
По технике |
|
|
По применению |
|
|
Конечным пользователем |
|
|
По каналу распространения |
|
Ожидается, что в 2025 году непрямая иммунофлуоресценция (НИФ) будет доминировать на рынке, занимая наибольшую долю в сегменте технологий.
Ожидается, что непрямая иммунофлуоресценция (ИИФ) будет доминировать на европейском рынке тестов на антинуклеарные антитела с наибольшей долей в 59,9% в 2025 году. Это доминирование в первую очередь обусловлено его статусом золотого стандарта для тестирования ANA, известного своей высокой чувствительностью и способностью обнаруживать широкий спектр аутоантител. Способность ИИФ определять различные паттерны окрашивания значительно помогает в диагностике множественных аутоиммунных заболеваний, что делает его предпочтительным выбором среди врачей. Постоянное совершенствование технологии ИИФ в сочетании с растущей распространенностью аутоиммунных заболеваний являются ключевыми факторами, определяющими его лидерство на рынке.
Ожидается, что извлекаемые ядерные антигены (ENA) составят наибольшую долю на рынке типов антител в течение прогнозируемого периода.
Ожидается, что в 2025 году сегмент извлекаемых ядерных антигенов (ENA) будет доминировать на рынке с наибольшей долей рынка в 34,7% из-за его высокой диагностической значимости при системных аутоиммунных ревматических заболеваниях (SARD), включая системную красную волчанку, синдром Шегрена и системную склеродермию. Способность панелей ENA одновременно обнаруживать несколько аутоантител повышает точность и эффективность диагностики. Повышение осведомленности среди врачей, достижения в технологиях мультиплексного анализа и растущая распространенность аутоиммунных заболеваний дополнительно поддерживают лидирующие позиции сегмента на рынке.
Региональный анализ европейского рынка тестов на антинуклеарные антитела
- Западная Европа занимает доминирующую долю европейского рынка тестов на антинуклеарные антитела, что обусловлено хорошо налаженной инфраструктурой здравоохранения, передовыми медицинскими технологиями и высоким спросом на специализированные диагностические решения. На Западную Европу приходится около 28% европейского рынка тестов на ANA
- Германия является ведущей страной в Европе с долей рынка 25,6% благодаря своей развитой системе здравоохранения, высокой осведомленности пациентов и наличию передовых технологий тестирования.
- Великобритания занимает значительную долю на европейском рынке тестов на антинуклеарные антитела, что объясняется надежной инфраструктурой здравоохранения и растущим спросом на специализированные диагностические тесты.
- Италия, как ожидается, испытает самый высокий совокупный годовой темп роста (CAGR) на рынке в 13,4% в течение прогнозируемого периода из-за роста аутоиммунных заболеваний, таких как волчанка, ревматоидный артрит и синдром Шегрена. Это растущее бремя болезней привело к более высокому спросу на раннее и точное диагностическое тестирование, включая тесты ANA
- Страны Восточной Европы, включая Польшу, Россию и Венгрию, переживают быстрый рост рынка из-за увеличения инвестиций в здравоохранение, повышения осведомленности и перехода к современным решениям в области здравоохранения. По оценкам, Восточная Европа будет вносить около 5–6% на европейский рынок тестов ANA
- Растущая тенденция домашнего здравоохранения в Европе, особенно в таких странах, как Нидерланды и Швеция, повышает спрос на услуги по тестированию ANA. Пациенты все чаще лечатся дома от хронических заболеваний, что стимулирует внедрение передовых диагностических инструментов. Диагностические решения, связанные с домашним здравоохранением в Европе, составляют около 4% европейского рынка тестов ANA
Доля рынка тестов на антинуклеарные антитела в Европе
Конкурентная среда рынка содержит сведения о конкурентах. Включены сведения о компании, ее финансах, полученном доходе, рыночном потенциале, инвестициях в исследования и разработки, новых рыночных инициативах, глобальном присутствии, производственных площадках и объектах, производственных мощностях, сильных и слабых сторонах компании, запуске продукта, широте и широте продукта, доминировании приложений. Приведенные выше данные касаются только фокуса компаний на рынке.
Основными лидерами рынка, работающими на рынке, являются:
- BioNTech SE (Германия)
- Genmab A/S (Дания)
- Evotec SE (Германия)
- Grifols SA . (Испания)
- CRISPR Therapeutics (Швейцария)
- Thermo Fisher Scientific Inc. (США)
- Trinity Biotech Ireland (Ирландия)
- EUROIMMUN Medizinische Labordiagnostika AG (Германия)
Последние разработки на европейском рынке тестов на антинуклеарные антитела
- В июне 2023 года компания EUROIMMUN Medizinische Labordiagnostika AG (Германия), входящая в состав Revvity, запустила UNIQO 160 — усовершенствованную автоматизированную систему непрямого иммунофлуоресцентного теста (IIFT). Эта система автоматизирует весь процесс IIFT — от подготовки образцов до анализа изображений — повышая эффективность и надежность диагностики аутоиммунных заболеваний.
- В мае 2023 года компания Thermo Fisher Scientific представила набор для аутоиммунного анализа, предназначенный для получения быстрых и точных результатов для выявления аутоиммунных заболеваний. Этот новый продукт направлен на улучшение диагностических возможностей и управления пациентами.
- В марте 2023 года Trinity Biotech запустила Autoimmune Panel Plus — диагностический анализ, обеспечивающий повышенную точность и скорость обнаружения различных аутоиммунных заболеваний. Этот продукт значительно улучшает диагностические рабочие процессы в клинических лабораториях.
- В июне 2022 года THERADIAG заключила партнерство с Quotient Limited для продвижения аутоиммунной диагностики с использованием платформы MosaiQ от Quotient. В рамках этого соглашения Theradiag поставляет Quotient аутоиммунные реагенты и средства контроля качества для разработки аутоиммунных микрочипов, причем первое приложение было сосредоточено на заболеваниях соединительной ткани (ЗСТ)
- В мае 2022 года компания ZEUS Scientific получила разрешение FDA на свою цифровую иммунофлуоресцентную систему dIFine, которая используется с непрямым флуоресцентным анализом антител (IFA) ANA HEp-2 от ZEUS. Это разрешение включает положительное и отрицательное определение и восемь общих схем окрашивания ANA HEp-2, что способствует повышению точности диагностики.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.