Рынок диагностики рака почки в Азиатско-Тихоокеанском регионе по типу теста (визуализация, тест на биомаркеры, анализ крови, биопсия, генетический тест и другие), стадия рака (стадия I, стадия II, стадия III и стадия IV), тип опухоли (почечно-клеточная карцинома, светлоклеточная почечно-клеточная карцинома, несветлоклеточная почечно-клеточная карцинома), продукт (платформенные продукты, продукты на основе инструментов, наборы и реагенты и другие расходные материалы), технология (флуоресцентная гибридизация in situ , секвенирование следующего поколения, флуоресцентный иммуноанализ, сравнительная геномная гибридизация, иммуногистохимия и другие), применение (скрининг, диагностика и предиктивный анализ, прогностический анализ и исследование), конечный пользователь (больницы, диагностические центры, онкологические исследовательские центры, академические институты, амбулаторные хирургические центры и другие), канал сбыта (прямой тендер, розничные продажи и другие), отраслевые тенденции и Прогноз до 2030 года.
Анализ и размер рынка диагностики рака почки в Азиатско-Тихоокеанском регионе
Рак почки начинается, когда здоровые клетки в одной или обеих почках изменяются и растут неконтролируемо, образуя массу, называемую кортикальной опухолью. Опухоль может быть злокачественной, вялотекущей или доброкачественной. Злокачественность — это рак, что означает, что она может расти и распространяться на другие части тела. Вялотекущая опухоль — это тоже рак, но этот тип опухоли редко распространяется на другие части тела. Доброкачественная опухоль означает, что опухоль может расти, но не распространяться.
Растущая осведомленность о раке почек в Азиатско-Тихоокеанском регионе увеличила спрос на рынке. Растущие расходы на здравоохранение для улучшения медицинских услуг также способствуют росту рынка. Основные игроки рынка сосредоточены на различных запусках услуг и одобрениях в этот критический период. Кроме того, рост улучшенных диагностических процедур рака почек также способствует росту спроса на диагностическое тестирование рака почек.
Ожидается, что рынок диагностики рака почки в Азиатско-Тихоокеанском регионе вырастет в прогнозируемом году за счет увеличения числа участников рынка и доступности передовых услуг. Наряду с этим производители занимаются НИОКР для запуска новых услуг на рынке. Ожидается, что растущие исследования в области диагностики и развития почек еще больше подстегнут рост рынка. Однако ожидается, что повреждение тканей из-за высокого уровня радиационного облучения при визуализационных тестах будет сдерживать рост рынка диагностики рака почки в Азиатско-Тихоокеанском регионе в прогнозируемый период.
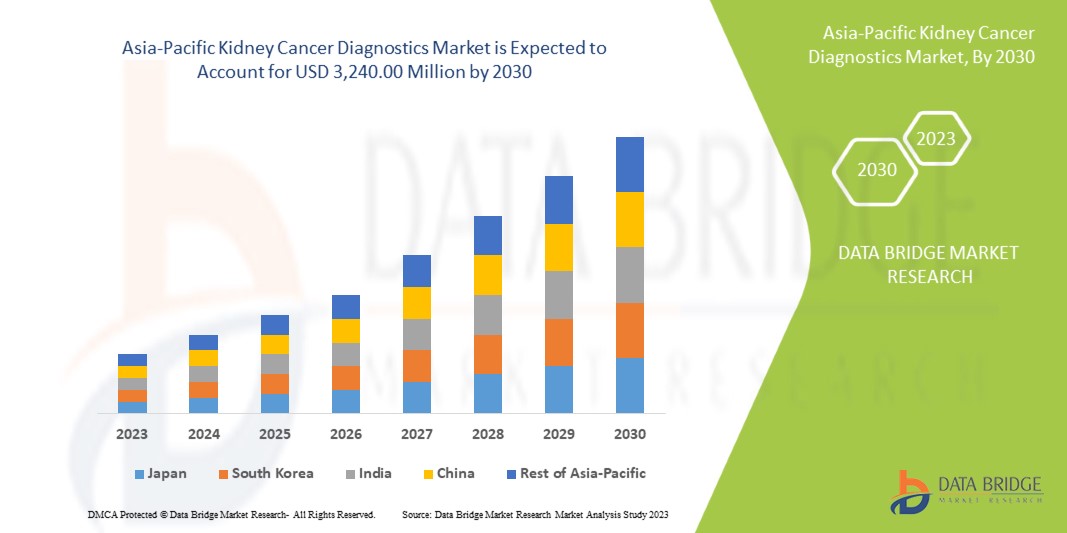
Data Bridge Market Research анализирует, что рынок диагностики рака почки, как ожидается, достигнет значения 3 240,00 млн долларов США к 2030 году при среднегодовом темпе роста 6,9% в течение прогнозируемого периода. Визуализация составляет крупнейший сегмент типа теста на рынке из-за растущего спроса на интеллектуальные устройства, а увеличение расходов на здравоохранение ускорило спрос на интеллектуальные медицинские устройства.
|
Отчет Метрика |
Подробности |
|
Прогнозируемый период |
2023-2030 |
|
Базовый год |
2022 |
|
Исторические годы |
2021 (можно настроить на 2020-2016) |
|
Количественные единицы |
Доход в млн. долл. США, объемы в единицах, цены в долл. США |
|
Охваченные сегменты |
По типу теста (визуализация, тест на биомаркеры, анализ крови, биопсия, генетический тест и другие), стадии рака (стадия I, стадия II, стадия III и стадия IV), типу опухоли (почечно-клеточный рак, светлоклеточный почечно-клеточный рак, несветлоклеточный почечно-клеточный рак), продукту (платформенные продукты, инструментальные продукты, наборы и реагенты и другие расходные материалы), технологии (флуоресцентная гибридизация in situ, секвенирование нового поколения, флуоресцентный иммуноанализ, сравнительная геномная гибридизация, иммуногистохимия и другие), применению (скрининг, диагностика и предиктивный анализ, прогностический анализ и исследование), конечному пользователю (больницы, диагностические центры, онкологические исследовательские центры, академические институты, амбулаторные хирургические центры и другие), каналу сбыта (прямой тендер, розничные продажи и другие). |
|
Страны, охваченные |
Китай, Япония, Индия, Австралия, Новая Зеландия, Южная Корея, Сингапур, Малайзия, Таиланд, Индонезия, Филиппины, Вьетнам, Тайвань и остальные страны Азиатско-Тихоокеанского региона. |
|
Охваченные участники рынка |
Siemens Healthcare GmbH, Koninklijke Philips NV, FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, Thermo Fisher Scientific, Myriad Genetics, Inc., CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Illumina Inc., Ambry Genetics, Invitae Corporation, General Electric Company, Centogene NV, GenPath, Creative Diagnostics, GeneDx LLC, Blueprint Genetics Oy, BioVendor R&D, CD Genomics и BD, среди прочих |
Определение рынка
Рак почки, обычно известный как рак почек, — это состояние, при котором клетки почек развиваются в злокачественные (раковые) опухоли и бесконтрольно разрастаются. Одним из 10 самых распространенных видов рака является рак почки. Рак почки смертелен, а процесс диагностики также имеет проблемы с безопасностью; он не является экономически эффективным. Больные раком могут быть госпитализированы и получать различные виды терапии, такие как хирургия, лучевая терапия и системная терапия. Около 40% новообразований почек представляют собой крошечные локализованные массы. Локализованные относятся к опухоли, которая не распространилась из своего первоначального местоположения. Массы почек невозможно обнаружить с помощью обычных лабораторных процедур. Диагностика рака почки включает процедуры биопсии, анализы крови и визуализационные тесты. Рекомендуются передовые методы лечения рака почки, такие как иммунотерапия, лучевая терапия и т. д. Благодаря передовым методам нехирургические процедуры, такие как криоабляция (замораживание раковых клеток) и радиочастотная абляция, иногда используются для лечения небольших опухолей почек (нагревание раковых клеток).
Рак почки может быть трудно диагностируемым, поскольку, несмотря на широкий спектр признаков и симптомов, они неспецифичны и могут быть связаны с другими, более распространенными заболеваниями. Более 43 000 мужчин и 25 000 женщин ежегодно диагностируются с раком почки и почечной лоханки, и 9 000 мужчин и 5 000 женщин умирают в результате этой болезни. Однако ожидается, что строгие правила и стандарты одобрения и коммерциализации диагностических продуктов для рака почки будут сдерживать рост рынка.
Динамика рынка диагностики рака почки в Азиатско-Тихоокеанском регионе
В этом разделе рассматривается понимание движущих сил рынка, преимуществ, возможностей, ограничений и проблем. Все это подробно обсуждается ниже:
Драйверы
- Растущая распространенность рака почки
Все возрасты могут быть затронуты этим типом рака. Рак почки может быть трудно диагностируемым, поскольку, несмотря на широкий спектр признаков и симптомов, они неспецифичны и могут быть связаны с другими, более распространенными заболеваниями. Рак почки обычно не имеет признаков или симптомов на ранних стадиях. Со временем могут появиться признаки и симптомы, включая кровь в моче, которая может казаться розовой, красной или цвета колы, боль в спине или боку, которая не проходит, потерю аппетита, необъяснимую потерю веса, усталость и лихорадку. У взрослых рак почки является наиболее распространенным типом рака. У маленьких детей чаще развивается тип рака почки, называемый опухолью Вильмса. Рак почки (также известный как рак почки или почечноклеточная аденокарцинома) является 14-м по распространенности раком в мире. Он занимает 9-е место среди мужчин и 14-е место среди женщин. В 2020 году было диагностировано более 30 000 новых случаев рака почки.
Из-за различных факторов риска, таких как курение, ожирение, высокое кровяное давление (гипертония) или семейный анамнез рака почки, число пациентов с раком почки растет в Азиатско-Тихоокеанском регионе и становится значительной социально-экономической проблемой. Таким образом, растущее число пациентов с раком почки увеличивает спрос на диагностические продукты для рака почки, которые выступают в качестве драйвера на рынке диагностики рака почки в Азиатско-Тихоокеанском регионе.
- Увеличение числа диагностических процедур при раке почки
Методы, используемые для диагностики рака почки, включают ультразвуковое исследование, компьютерную томографию (КТ), магнитно-резонансную томографию (МРТ) и иногда позитронно-эмиссионную томографию (ПЭТ). Лечение рака почки с медленным темпом роста может включать мониторинг. Химиотерапию при злокачественных опухолях иногда сочетают с лучевой терапией и пересадкой стволовых клеток. Рост заболеваемости раком стал фактором, способствующим увеличению одобрения диагностических продуктов.
Таким образом, рост числа одобрений диагностических продуктов привел к увеличению числа высокоэффективных продуктов на рынке диагностики и лечения рака почки. Ожидается, что это станет драйвером роста рынка диагностики рака почки в Азиатско-Тихоокеанском регионе.
Возможность
-
Растущее предпочтение профилактическим медицинским осмотрам
Профилактический медицинский осмотр — это профилактические действия, выполняемые для первоначального выявления заболевания раком почек. Кроме того, растущая популярность профилактических медицинских осмотров обеспечивает защиту от вероятного воздействия любого заболевания в будущем.
Осведомленность о необходимости проведения скрининга является важнейшим компонентом профилактики рака почек. Проверка включает в себя выявление рака и изучение факторов риска для ограничения потерь на ранней стадии.
Профилактическое обследование на предмет рака почки проводится с помощью различных диагностических тестов, включающих биопсию, иммуногистохимию, скрининг рака, МРТ и другие.
Люди относительно более подвержены заболеваниям рака почек. Поэтому им необходимо проходить регулярные осмотры, чтобы помочь врачам развить понимание заболеваний и обеспечить лучшее лечение для пациента, страдающего от рака, по той причине, что растущее предпочтение профилактическим осмотрам, как ожидается, станет движущей силой роста рынка диагностики рака почек в Азиатско-Тихоокеанском регионе.
Сдержанность/Вызов
- Строгие правила и стандарты для одобрения и коммерциализации продуктов диагностики рака почки
Строгие правила коммерциализации любого продукта на рынке становятся серьезной проблемой для производителей средств диагностики рака в Азиатско-Тихоокеанском регионе, у которых есть свои правила и другой орган для осуществления нормативных процедур.
Производители сначала должны проверить одобрение маркировки CE для коммерциализации своего продукта на рынке Азиатско-Тихоокеанского региона. Ожидается, что строгая нормативная политика будет препятствовать развитию рынка диагностики рака. Нормативные требования к одобрению маркетинга или сертификации CE и применению законов и правил могут привести к серьезным изменениям в бизнесе или выплате штрафов, включая потенциальную потерю лицензий на ведение бизнеса. Ресурсы и затраты, необходимые для соблюдения этих законов, правил и положений, высоки.
Нормативные требования к маркетинговым разрешениям, декларации соответствия и время, необходимое для нормативного обзора, могут различаться для разных продуктов. Компания, которая не получает нормативного одобрения, наносит вред бизнесу, поскольку без получения одобрения или без получения одобрения CE на продукцию производители не могут выпустить свою продукцию на рынок Азиатско-Тихоокеанского региона, и по этой причине ожидается, что строгие правила и стандарты для одобрения и коммерциализации диагностических продуктов для рака почки будут действовать как сдерживающий фактор для рынка диагностики рака почки в Азиатско-Тихоокеанском регионе.
Последние события
- В ноябре 2022 года компания Koninklijke Philips NV объявила о запуске в Азиатско-Тихоокеанском регионе компактного портативного ультразвукового решения следующего поколения на ежегодном собрании Радиологического общества Северной Америки (RSNA), чтобы предоставить большему количеству пациентов диагностическое качество, связанное с премиальными ультразвуковыми системами на тележке. Оно портативное и универсальное с хорошим качеством изображения или производительностью. Оно совместимо с ультразвуковыми системами Philips Affiniti и датчиком EPIQ. Это помогло компании расширить свой ассортимент продукции.
- В октябре 2022 года компания General Electric сотрудничала с несколькими научно-исследовательскими институтами, такими как University of Cambridge Hospitals, Sophia Genetics, а ранее с Optellum, для использования данных визуализации в сотрудничестве с искусственным интеллектом. Это поможет сократить время диагностики некоторых видов рака и предоставить пациентам персонализированную помощь. Это помогло компании расширить свои горизонты в диагностике рака.
- В июле 2022 года компания Canon Medical Systems USA Inc. объявила о завершении сделки по приобретению компании NXC Imaging, дистрибьютора и поставщика услуг медицинского диагностического оборудования, расположенного в Миннесоте, США. Это привело к расширению охвата услуг на рынке Азиатско-Тихоокеанского региона.
Масштаб рынка диагностики рака почки в Азиатско-Тихоокеанском регионе
Рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментирован на восемь заметных сегментов на основе типа теста, стадии рака, типа опухоли, продукта, применения, технологии, конечного пользователя и канала сбыта. Рост среди сегментов помогает вам анализировать нишевые карманы роста и стратегии для выхода на рынок и определять ваши основные области применения и разницу в ваших целевых рынках.
Тип теста
- ТЕСТИРОВАНИЕ ИЗОБРАЖЕНИЯ
- ТЕСТ НА БИОМАРКЕРЫ
- АНАЛИЗ КРОВИ
- БИОПСИЯ
- ГЕНЕТИЧЕСКИЙ ТЕСТ
- ДРУГИЕ
По типу теста рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментируется на визуализацию, тесты на биомаркеры, анализы крови, биопсию, генетические тесты и другие.
Стадия рака
- ЭТАП I
- ЭТАП II
- ЭТАП 3
- ЭТАП IV
В зависимости от стадии рака рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментирован на стадию I, стадию II, стадию III и стадию IV.
Тип опухоли
- ПОЧЕЧНО-КЛЕТОЧНЫЙ РАК
- Светлоклеточная почечноклеточная карцинома
- НЕСВЕТЛОКЛЕТОЧНАЯ ПОЧЕЧНО-КЛЕТОЧНАЯ КАРЦИНОМА
В зависимости от типа опухоли рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментируется на почечно-клеточный рак, светлоклеточный почечно-клеточный рак и несветлоклеточный почечно-клеточный рак.
Продукт
- ПРОДУКТЫ НА ОСНОВЕ ПЛАТФОРМЫ
- ИНСТРУМЕНТАЛЬНЫЕ ПРОДУКТЫ
- НАБОРЫ И РЕАГЕНТЫ
- ДРУГИЕ РАСХОДНЫЕ МАТЕРИАЛЫ
По видам продукции рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментируется на инструментальную продукцию, платформенную продукцию, наборы и реагенты, а также другие расходные материалы.
Технологии
- ФЛУОРЕСЦЕНТНАЯ ГИБРИДИЗАЦИЯ IN SITU
- СЕКВЕНИРОВАНИЕ СЛЕДУЮЩЕГО ПОКОЛЕНИЯ
- ФТОРИММУНОАНАЛИЗ
- СРАВНИТЕЛЬНАЯ ГЕНОМНАЯ ГИБРИДИЗАЦИЯ
- ИММУНОГИСТОХИМИЧЕСКИЙ
- ДРУГИЕ
На основе технологий рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментирован на флуоресцентную гибридизацию in situ, секвенирование нового поколения, флуоресцентный иммуноанализ, сравнительную геномную гибридизацию, иммуногистохимию и другие.
Приложение
- СКРИНИНГ
- ДИАГНОСТИЧЕСКИЙ И ПРОГНОСТИЧЕСКИЙ
- ПРОГНОСТИЧЕСКИЙ
- ИССЛЕДОВАТЬ
По сфере применения рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментируется на скрининговый, диагностико-предиктивный, прогностический и исследовательский.
Конечный пользователь
- БОЛЬНИЦЫ
- ЦЕНТРЫ ИССЛЕДОВАНИЯ РАКА
- АКАДЕМИЧЕСКИЕ ИНСТИТУТЫ
- ДИАГНОСТИЧЕСКИЕ ЦЕНТРЫ
- АМБУЛАТОРНЫЕ ХИРУРГИЧЕСКИЕ ЦЕНТРЫ
- ДРУГИЕ
По типу конечных пользователей рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментирован на больницы, диагностические центры, центры исследований рака, академические институты, амбулаторные хирургические центры и другие.
Канал распространения
- ПРЯМЫЕ ТЕНДЕРЫ
- РОЗНИЧНЫЕ ПРОДАЖИ
- ДРУГИЕ
По каналам сбыта рынок диагностики рака почки в Азиатско-Тихоокеанском регионе сегментируется на прямые торги, розничные продажи и другие.
Региональный анализ/информация о рынке диагностики рака почки в Азиатско-Тихоокеанском регионе
Проведен анализ рынка диагностики рака почки в Азиатско-Тихоокеанском регионе и предоставлена информация о размере рынка с учетом страны, типа теста, стадии рака, типа опухоли, продукта, области применения, технологии, конечного пользователя и канала сбыта.
В данном отчете о рынке рассматриваются следующие страны: Китай, Япония, Индия, Австралия, Новая Зеландия, Южная Корея, Сингапур, Малайзия, Таиланд, Индонезия, Филиппины, Вьетнам, Тайвань и остальные страны Азиатско-Тихоокеанского региона.
Китай доминирует в Азиатско-Тихоокеанском регионе благодаря растущей осведомленности о необходимости регулярных медицинских осмотров.
Раздел отчета по странам также содержит отдельные факторы, влияющие на рынок, и изменения в регулировании на внутреннем рынке, которые влияют на текущие и будущие тенденции рынка. Такие данные, как новые продажи, заменяющие продажи, демографические данные страны, нормативные акты и импортно-экспортные тарифы, являются одними из основных указателей, используемых для прогнозирования рыночного сценария для отдельных стран. Кроме того, при предоставлении прогнозного анализа данных по странам учитываются наличие и доступность брендов Азиатско-Тихоокеанского региона и их проблемы из-за большой или малой конкуренции со стороны местных и отечественных брендов, а также влияние каналов продаж.
Анализ конкурентной среды и доли рынка диагностики рака почки в Азиатско-Тихоокеанском регионе
Конкурентная среда рынка диагностики рака почки содержит сведения по конкурентам. Включены сведения о компании, ее финансах, полученном доходе, рыночном потенциале, инвестициях в НИОКР, новых рыночных инициативах, производственных площадках и объектах, сильных и слабых сторонах компании, запуске продукта, одобрении продукта, широте и широте продукта, доминировании приложений и жизненно важной кривой типа продукта. Приведенные выше данные касаются только фокуса компании на рынке диагностики рака почки.
Некоторые из основных игроков, работающих на рынке, включают Siemens Healthcare GmbH, Koninklijke Philips NV, FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, Thermo Fisher Scientific, Myriad Genetics, Inc., CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Illumina Inc., Ambry Genetics, Invitae Corporation, General Electric Company, Centogene NV, GenPath, Creative Diagnostics, GeneDx LLC, Blueprint Genetics Oy, BioVendor R&D и CD Genomics, BD и другие.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Содержание
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 TEST TYPE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
4.3 EPIDEMIOLOGY
4.3.1 KIDNEY CANCER INCIDENCES, 2020, BY BOTH SEXES
4.3.2 KIDNEY CANCER MORTALITY, 2020, BY BOTH SEXES
5 INDUSTRY INSIGHTS
6 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING PREVALENCE OF KIDNEY CANCER
7.1.2 INCREASE IN DIAGNOSTIC PROCEDURES FOR KIDNEY CANCER
7.1.3 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.1.4 RISING AWARENESS TOWARDS KIDNEY CANCER
7.2 RESTRAINTS
7.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF KIDNEY CANCER DIAGNOSTIC PRODUCTS
7.2.2 TISSUE DAMAGE DUE TO HIGH RADIATION EXPOSURE FROM IMAGING TESTS
7.3 OPPORTUNITIES
7.3.1 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
7.3.2 GOVERNMENT INITIATIVES TOWARD KIDNEY CANCER DIAGNOSTICS
7.3.3 GROWING DEMAND FOR BETTER QUALITY HEALTHCARE
7.3.4 INCREASED DEMAND FOR NON-INVASIVE TESTING METHODS
7.4 CHALLENGES
7.4.1 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
7.4.2 HIGH COST OF DIAGNOSTICS PROCEDURE FOR KIDNEY CANCERS
8 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 IMAGING
8.2.1 COMPUTED TOMOGRAPHY
8.2.2 ULTRASOUND
8.2.3 MAGNETIC RESONANCE IMAGING (MRI)
8.2.4 ANGIOGRAPHY
8.2.5 X-RAY
8.2.6 OTHERS
8.3 BLOOD TEST
8.4 BIOPSY
8.4.1 FINE NEEDLE ASPIRATION
8.4.2 NEEDLE CORE BIOPSY
8.5 BIOMARKER TEST
8.5.1 AQUAPORIN 1 (AQP1)
8.5.2 PERILIPIN (PLIN2)
8.5.3 N-METHYLTRANSFERASE (NMNT)
8.5.4 L-PLASTIN (LCP-1)
8.5.5 NM23A
8.6 GENETIC TEST
8.7 OTHERS
9 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE
9.1 OVERVIEW
9.2 STAGE I
9.3 STAGE II
9.4 STAGE III
9.5 STAGE IV
10 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE
10.1 OVERVIEW
10.2 RENAL CELL CARCINOMA
10.2.1 IMAGING
10.2.2 BLOOD TEST
10.2.3 BIOPSY
10.2.4 BIOMARKER TEST
10.2.5 GENETIC TEST
10.2.6 OTHERS
10.3 CLEAR CELL RENAL CELL CARCINOMA
10.3.1 IMAGING
10.3.2 BLOOD TEST
10.3.3 BIOPSY
10.3.4 BIOMARKER TEST
10.3.5 GENETIC TEST
10.3.6 OTHERS
10.4 NON CLEAR CELL RENAL CELL CARCINOMA
10.4.1 PAPILLARY RENAL CELL CARCINOMA
10.4.1.1 IMAGING
10.4.1.2 BLOOD TEST
10.4.1.3 BIOPSY
10.4.1.4 BIOMARKER TEST
10.4.1.5 GENETIC TEST
10.4.1.6 OTHERS
10.4.2 CHROMOPHOBE RENAL CELL CARCINOMA
10.4.2.1 IMAGING
10.4.2.2 BLOOD TEST
10.4.2.3 BIOPSY
10.4.2.4 BIOMARKER TEST
10.4.2.5 GENETIC TEST
10.4.2.6 OTHERS
11 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT
11.1 OVERVIEW
11.2 INSTRUMENT BASED PRODUCTS
11.2.1 IMAGING
11.2.2 BIOPSY
11.3 PLATFORM BASED PRODUCTS
11.3.1 NEXT GENERATION SEQUENCING
11.3.2 MICROARRAYS
11.3.3 PCR
11.3.4 OTHERS
11.4 KITS AND REAGENTS
11.4.1 RENAL CANCER PANELS
11.4.2 RENAL CANCER ANTIBODIES
11.5 OTHER CONSUMABLES
12 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY
12.1 OVERVIEW
12.2 FLUORESCENT IN SITU HYBRIDIZATION
12.3 NEXT GENERATION SEQUENCING
12.4 FLUORIMMUNOASSAY
12.5 COMPARATIVE GENOMIC HYBRIDIZATION
12.6 IMMUNOHISTOCHEMICAL
12.7 OTHERS
13 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION
13.1 OVERVIEW
13.2 SCREENING
13.2.1 INSTRUMENT BASED PRODUCTS
13.2.2 PLATFORM BASED PRODUCTS
13.2.3 KITS AND REAGENTS
13.2.4 OTHER CONSUMABLES
13.3 DIAGNOSTIC AND PREDICTIVE
13.3.1 INSTRUMENT BASED PRODUCTS
13.3.2 PLATFORM BASED PRODUCTS
13.3.3 KITS AND REAGENTS
13.3.4 OTHER CONSUMABLES
13.4 PROGNOSTIC
13.4.1 INSTRUMENT BASED PRODUCTS
13.4.2 PLATFORM BASED PRODUCTS
13.4.3 KITS AND REAGENTS
13.4.4 OTHER CONSUMABLES
13.5 RESEARCH
13.5.1 INSTRUMENT BASED PRODUCTS
13.5.2 PLATFORM BASED PRODUCTS
13.5.3 KITS AND REAGENTS
13.5.4 OTHER CONSUMABLES
14 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITALS
14.3 DIAGNOSTIC CENTERS
14.4 CANCER RESEARCH CENTERS
14.5 ACADEMIC INSTITUTES
14.6 AMBULATORY SURGICAL CENTERS
14.7 OTHERS
15 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
15.4 OTHERS
16 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION
16.1 ASIA-PACIFIC
16.1.1 CHINA
16.1.2 JAPAN
16.1.3 INDIA
16.1.4 SOUTH KOREA
16.1.5 AUSTRALIA
16.1.6 TAIWAN
16.1.7 NEW ZEALAND
16.1.8 INDONESIA
16.1.9 THAILAND
16.1.10 PHILIPPINES
16.1.11 MALAYSIA
16.1.12 VIETNAM
16.1.13 SINGAPORE
16.1.14 REST OF ASIA PACIFIC
17 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 CANON MEDICAL SYSTEMS CORPORATION
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY PROFILE
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 KONINKLIJKE PHILIPS N.V.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY PROFILE
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 GENERAL ELECTRIC COMPANY
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY PROFILE
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.4 SIEMENS HEALTHCARE GMBH
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY PROFILE
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENT
19.5 GRAIL
19.5.1 COMPANY SNAPSHOT
19.5.2 PRODUCT PORTFOLIO
19.5.3 RECENT DEVELOPMENTS
19.6 AMBRY GENETICS
19.6.1 COMPANY SNAPSHOT
19.6.2 PRODUCT PORTFOLIO
19.6.3 RECENT DEVELOPMENT
19.7 BIOVENDOR R&D
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 BLUEPRINT GENETICS OY.
19.8.1 COMPANY SNAPSHOT
19.8.2 PRODUCT PORTFOLIO
19.8.3 RECENT DEVELOPMENT
19.9 CD GENOMICS
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENT
19.1 CENTOGENE N.V.
19.10.1 COMPANY SNAPSHOT
19.10.2 REVENUE ANALYSIS
19.10.3 PRODUCT PORTFOLIO
19.10.4 RECENT DEVELOPMENT
19.11 CREATIVE DIAGNOSTICS
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 FUJIFILM CORPORATION
19.12.1 COMPANY SNAPSHOT
19.12.2 REVENUE ANALYSIS
19.12.3 PRODUCT PORTFOLIO
19.12.4 RECENT DEVELOPMENTS
19.13 GENEDX, LLC
19.13.1 COMPANY SNAPSHOT
19.13.2 REVENUE ANALYSIS
19.13.3 PRODUCT PORTFOLIO
19.13.4 RECENT DEVELOPMENT
19.14 GENPATH, A DIVISION OF BIOREFERENCE LABORATORIES, AN OPKO HEALTH INC. COMPANY
19.14.1 COMPANY SNAPSHOT
19.14.2 PRODUCT PORTFOLIO
19.14.3 RECENT DEVELOPMENT
19.15 ILLUMINA, INC.
19.15.1 COMPANY SNAPSHOT
19.15.2 REVENUE ANALYSIS
19.15.3 PRODUCT PORTFOLIO
19.15.4 RECENT DEVELOPMENT
19.16 INVITAE CORPORATION
19.16.1 COMPANY SNAPSHOT
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.17 LABORATORY CORPORATION OF AMERICA HOLDINGS
19.17.1 COMPANY SNAPSHOT
19.17.2 REVENUE ANALYSIS
19.17.3 PRODUCT PORTFOLIO
19.17.4 RECENT DEVELOPMENTS
19.18 MYRIAD GENETICS, INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
19.19 THERMO FISHER SCIENTIFIC INC.
19.19.1 COMPANY SNAPSHOT
19.19.2 REVENUE ANALYSIS
19.19.3 PRODUCT PORTFOLIO
19.19.4 RECENT DEVELOPMENT
19.2 QIAGEN
19.20.1 COMPANY SNAPSHOT
19.20.2 REVENUE ANALYSIS
19.20.3 PRODUCT PORTFOLIO
19.20.4 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
Список таблиц
TABLE 1 ESTIMATED NEW CANCER CASES AND DEATHS
TABLE 2 APPROVED DIAGNOSTICS OF KIDNEY CANCER
TABLE 3 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 4 ASIA PACIFIC IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 ASIA PACIFIC IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 ASIA PACIFIC BLOOD TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 ASIA PACIFIC BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 ASIA PACIFIC BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 9 ASIA PACIFIC BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 ASIA PACIFIC BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 11 ASIA PACIFIC GENETIC TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 ASIA PACIFIC OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 14 ASIA PACIFIC STAGE I IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 ASIA PACIFIC STAGE II IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 ASIA PACIFIC STAGE III IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 ASIA PACIFIC STAGE IV IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 19 ASIA PACIFIC RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 ASIA PACIFIC RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 21 ASIA PACIFIC CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 ASIA PACIFIC CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 23 ASIA PACIFIC NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 ASIA PACIFIC NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 25 ASIA PACIFIC PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 26 ASIA PACIFIC CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 27 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 ASIA PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 ASIA PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 30 ASIA PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 31 ASIA PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 32 ASIA PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 ASIA PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 34 ASIA PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 35 ASIA PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 36 ASIA PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 ASIA PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 38 ASIA PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 39 ASIA PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 40 ASIA PACIFIC OTHER CONSUMABLES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 42 ASIA PACIFIC FLUORESCENT IN SITU HYBRIDIZATION IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 ASIA PACIFIC NEXT GENERATION SEQUENCING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 ASIA PACIFIC FLUORIMMUNOASSAY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 ASIA PACIFIC COMPARATIVE GENOMIC HYBRIDIZATION IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 ASIA PACIFIC IMMUNOHISTOCHEMICAL IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 ASIA PACIFIC OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 48 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 49 ASIA PACIFIC SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 ASIA PACIFIC SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 51 ASIA PACIFIC DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 52 ASIA PACIFIC DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 53 ASIA PACIFIC PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 54 ASIA PACIFIC PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 55 ASIA PACIFIC RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 56 ASIA PACIFIC RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 57 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 58 ASIA PACIFIC HOSPITALS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 ASIA PACIFIC DIAGNOSTIC CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 60 ASIA PACIFIC CANCER RESEARCH CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 61 ASIA PACIFIC ACADEMIC INSTITUTES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 62 ASIA PACIFIC AMBULATORY SURGICAL CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 ASIA PACIFIC OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 65 ASIA PACIFIC DIRECT TENDER IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 66 ASIA PACIFIC RETAIL SALES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 67 ASIA PACIFIC OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 68 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 69 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 ASIA-PACIFIC IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 ASIA-PACIFIC BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 ASIA-PACIFIC BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 74 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 75 ASIA-PACIFIC RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 76 ASIA-PACIFIC CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 77 ASIA-PACIFIC NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 78 ASIA-PACIFIC PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 79 ASIA-PACIFIC CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 80 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 ASIA-PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 ASIA-PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 83 ASIA-PACIFIC INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 84 ASIA-PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 ASIA-PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 86 ASIA-PACIFIC PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 87 ASIA-PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 ASIA-PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 ASIA-PACIFIC KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 92 ASIA-PACIFIC SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 ASIA-PACIFIC DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 94 ASIA-PACIFIC PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 95 ASIA-PACIFIC RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 CHINA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 CHINA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 CHINA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 102 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 103 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 104 CHINA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 CHINA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 CHINA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 CHINA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 108 CHINA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 109 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 110 CHINA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 111 CHINA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 112 CHINA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 113 CHINA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 114 CHINA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 115 CHINA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 116 CHINA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 CHINA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 118 CHINA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 119 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 120 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 121 CHINA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 CHINA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 123 CHINA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 124 CHINA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 126 CHINA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 127 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 128 JAPAN IMAGING TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 129 JAPAN BIOPSY TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 130 JAPAN BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 131 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 132 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 133 JAPAN RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 JAPAN CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 JAPAN NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 136 JAPAN PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 137 JAPAN CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 139 JAPAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 140 JAPAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 141 JAPAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 142 JAPAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 143 JAPAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 144 JAPAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 145 JAPAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 146 JAPAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 147 JAPAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 148 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 149 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 150 JAPAN SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 151 JAPAN DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 JAPAN PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 JAPAN RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 155 JAPAN KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 156 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 157 INDIA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 158 INDIA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 159 INDIA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 160 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 161 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 162 INDIA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 INDIA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 164 INDIA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 165 INDIA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 INDIA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 168 INDIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 169 INDIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 170 INDIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 171 INDIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 172 INDIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 173 INDIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 174 INDIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 175 INDIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 176 INDIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 177 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 178 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 179 INDIA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 180 INDIA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 181 INDIA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 182 INDIA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 183 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 184 INDIA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 185 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 186 SOUTH KOREA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 187 SOUTH KOREA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 188 SOUTH KOREA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 189 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 190 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 191 SOUTH KOREA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 192 SOUTH KOREA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 193 SOUTH KOREA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 194 SOUTH KOREA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 195 SOUTH KOREA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 196 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 197 SOUTH KOREA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 198 SOUTH KOREA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 199 SOUTH KOREA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 200 SOUTH KOREA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 201 SOUTH KOREA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 202 SOUTH KOREA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 203 SOUTH KOREA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 204 SOUTH KOREA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 205 SOUTH KOREA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 206 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 207 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 208 SOUTH KOREA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 209 SOUTH KOREA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 210 SOUTH KOREA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 211 SOUTH KOREA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 212 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 213 SOUTH KOREA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 214 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 215 AUSTRALIA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 216 AUSTRALIA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 217 AUSTRALIA BIOMAKER IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 218 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 219 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 220 AUSTRALIA RENAL CELL CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 221 AUSTRALIA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 222 AUSTRALIA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 223 AUSTRALIA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 224 AUSTRALIA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 225 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 226 AUSTRALIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 227 AUSTRALIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 228 AUSTRALIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 229 AUSTRALIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 230 AUSTRALIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 231 AUSTRALIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 232 AUSTRALIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 233 AUSTRALIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 234 AUSTRALIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 235 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 236 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 237 AUSTRALIA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 238 AUSTRALIA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 239 AUSTRALIA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 240 AUSTRALIA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 241 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 242 AUSTRALIA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 243 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 244 TAIWAN IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 245 TAIWAN BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 246 TAIWAN BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 247 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 248 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 249 TAIWAN RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 250 TAIWAN CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 251 TAIWAN NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 252 TAIWAN PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 253 TAIWAN CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 254 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 255 TAIWAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 256 TAIWAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 257 TAIWAN INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 258 TAIWAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 259 TAIWAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 260 TAIWAN PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 261 TAIWAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 262 TAIWAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 263 TAIWAN KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 264 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 265 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 266 TAIWAN SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 267 TAIWAN DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 268 TAIWAN PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 269 TAIWAN RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 270 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 271 TAIWAN KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 272 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 273 NEW ZEALAND IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 274 NEW ZEALAND BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 275 NEW ZEALAND BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 276 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 277 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 278 NEW ZEALAND RENAL CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 279 NEW ZEALAND CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 280 NEW ZEALAND NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 281 NEW ZEALAND PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 282 NEW ZEALAND CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 283 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 284 NEW ZEALAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 285 NEW ZEALAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 286 NEW ZEALAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 287 NEW ZEALAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 288 NEW ZEALAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 289 NEW ZEALAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 290 NEW ZEALAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 291 NEW ZEALAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 292 NEW ZEALAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 293 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 294 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 295 NEW ZEALAND SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 296 NEW ZEALAND DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 297 NEW ZEALAND PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 298 NEW ZEALAND RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 299 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 300 NEW ZEALAND KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 301 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 302 INDONESIA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 303 INDONESIA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 304 INDONESIA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 305 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 306 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 307 INDONESIA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 308 INDONESIA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 309 INDONESIA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 310 INDONESIA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 311 INDONESIA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 312 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 313 INDONESIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 314 INDONESIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 315 INDONESIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 316 INDONESIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 317 INDONESIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 318 INDONESIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 319 INDONESIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 320 INDONESIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 321 INDONESIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 322 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 323 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 324 INDONESIA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 325 INDONESIA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 326 INDONESIA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 327 INDONESIA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 328 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 329 INDONESIA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 330 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 331 THAILAND IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 332 THAILAND BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 333 THAILAND BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 334 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 335 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 336 THAILAND RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 337 THAILAND CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 338 THAILAND NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 339 THAILAND PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 340 THAILAND CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 341 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 342 THAILAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 343 THAILAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 344 THAILAND INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 345 THAILAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 346 THAILAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 347 THAILAND PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 348 THAILAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 349 THAILAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 350 THAILAND KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 351 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 352 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 353 THAILAND SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 354 THAILAND DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 355 THAILAND PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 356 THAILAND RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 357 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 358 THAILAND KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 359 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 360 PHILIPPINES IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 361 PHILIPPINES BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 362 PHILIPPINES BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 363 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 364 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 365 PHILIPPINES RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 366 PHILIPPINES CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 367 PHILIPPINES NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 368 PHILIPPINES PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 369 PHILIPPINES CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 370 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 371 PHILIPPINES INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 372 PHILIPPINES INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 373 PHILIPPINES INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 374 PHILIPPINES PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 375 PHILIPPINES PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 376 PHILIPPINES PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 377 PHILIPPINES KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 378 PHILIPPINES KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 379 PHILIPPINES KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 380 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 381 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 382 PHILIPPINES SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 383 PHILIPPINES DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 384 PHILIPPINES PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 385 PHILIPPINES RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 386 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 387 PHILIPPINES KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 388 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 389 MALAYSIA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 390 MALAYSIA BIOPSY TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 391 MALAYSIA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 392 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 393 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 394 MALAYSIA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 395 MALAYSIA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 396 MALAYSIA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 397 MALAYSIA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 398 MALAYSIA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 399 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 400 MALAYSIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 401 MALAYSIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 402 MALAYSIA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 403 MALAYSIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 404 MALAYSIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 405 MALAYSIA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 406 MALAYSIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 407 MALAYSIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 408 MALAYSIA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 409 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 410 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 411 MALAYSIA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 412 MALAYSIA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 413 MALAYSIA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 414 MALAYSIA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 415 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 416 MALAYSIA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 417 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 418 VIETNAM IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 419 VIETNAM BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 420 VIETNAM BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 421 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 422 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 423 VIETNAM RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 424 VIETNAM CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 425 VIETNAM NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 426 VIETNAM PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 427 VIETNAM CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 428 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 429 VIETNAM INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 430 VIETNAM INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 431 VIETNAM INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 432 VIETNAM PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 433 VIETNAM PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 434 VIETNAM PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 435 VIETNAM KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 436 VIETNAM KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 437 VIETNAM KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 438 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 439 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 440 VIETNAM SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 441 VIETNAM DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 442 VIETNAM PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 443 VIETNAM RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 444 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 445 VIETNAM KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 446 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 447 SINGAPORE IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 448 SINGAPORE BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 449 SINGAPORE BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 450 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 451 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 452 SINGAPORE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 453 SINGAPORE CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 454 SINGAPORE NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 455 SINGAPORE PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 456 SINGAPORE CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 457 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 458 SINGAPORE INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 459 SINGAPORE INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 460 SINGAPORE INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 461 SINGAPORE PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 462 SINGAPORE PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 463 SINGAPORE PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 464 SINGAPORE KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 465 SINGAPORE KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 466 SINGAPORE KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 467 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 468 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 469 SINGAPORE SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 470 SINGAPORE DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 471 SINGAPORE PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 472 SINGAPORE RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 473 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 474 SINGAPORE KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 475 REST OF ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
Список рисунков
FIGURE 1 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF KIDNEY CANCER AND INCREASING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE GROWTH OF THE ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 THE IMAGING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET
FIGURE 14 KIDNEY CANCER INCIDENCE, BOTH SEXES, BY REGION (2020)
FIGURE 15 KIDNEY CANCER MORTALITY, BOTH SEXES, BY REGION (2020)
FIGURE 16 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2022
FIGURE 17 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 18 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, CAGR (2023-2030)
FIGURE 19 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, LIFELINE CURVE
FIGURE 20 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE , 2022
FIGURE 21 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, 2023-2030 (USD MILLION)
FIGURE 22 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, CAGR (2023-2030)
FIGURE 23 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, LIFELINE CURVE
FIGURE 24 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, 2022
FIGURE 25 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 26 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 27 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, LIFELINE CURVE
FIGURE 28 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, 2022
FIGURE 29 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 30 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, CAGR (2023-2030)
FIGURE 31 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, LIFELINE CURVE
FIGURE 32 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, 2022
FIGURE 33 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 34 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 35 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, LIFELINE CURVE
FIGURE 36 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, 2022
FIGURE 37 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 38 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, CAGR (2023-2030)
FIGURE 39 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, LIFELINE CURVE
FIGURE 40 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, 2022
FIGURE 41 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, 2023-2030 (USD MILLION)
FIGURE 42 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, CAGR (2023-2030)
FIGURE 43 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, LIFELINE CURVE
FIGURE 44 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2022
FIGURE 45 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 46 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 47 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 49 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 50 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 51 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 52 ASIA-PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: TEST TYPE (2023-2030)
FIGURE 53 ASIA PACIFIC KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.