Рынок аутсорсинга вопросов регулирования диагностики in vitro в Азиатско-Тихоокеанском регионе по услугам (написание и подача нормативных документов, регистрация нормативных документов и заявки на клинические испытания, консалтинг по нормативным вопросам, юридическое представительство, услуги по управлению данными, услуги по производству и контролю химии (CMC) и другие), показания (онкология, неврология , кардиология, клиническая химия и иммуноанализы, прецизионная медицина , инфекционные заболевания, диабет, генетическое тестирование, ВИЧ/СПИД, гематология, тестирование на наркотики/фармакогеномика, переливание крови, точка оказания медицинской помощи и другие), модель развертывания (облачная и локальная), размер организации (малые и средние предприятия (SMES) и крупные предприятия), стадия (клиническая, доклиническая и PMA (пострегистрационная авторизация)), класс (класс I, класс II и класс III), конечный пользователь (фармацевтические компании, компании по производству медицинских приборов, биотехнологии) Компании и другие), страны (Китай, Южная Корея, Япония, Индия, Австралия, Сингапур, Малайзия, Индонезия, Таиланд, Филиппины и остальные страны Азиатско-Тихоокеанского региона). Отраслевые тенденции и прогноз до 2029 года.
Анализ рынка и аналитика : Азиатско-Тихоокеанский рынок аутсорсинга вопросов регулирования IVD
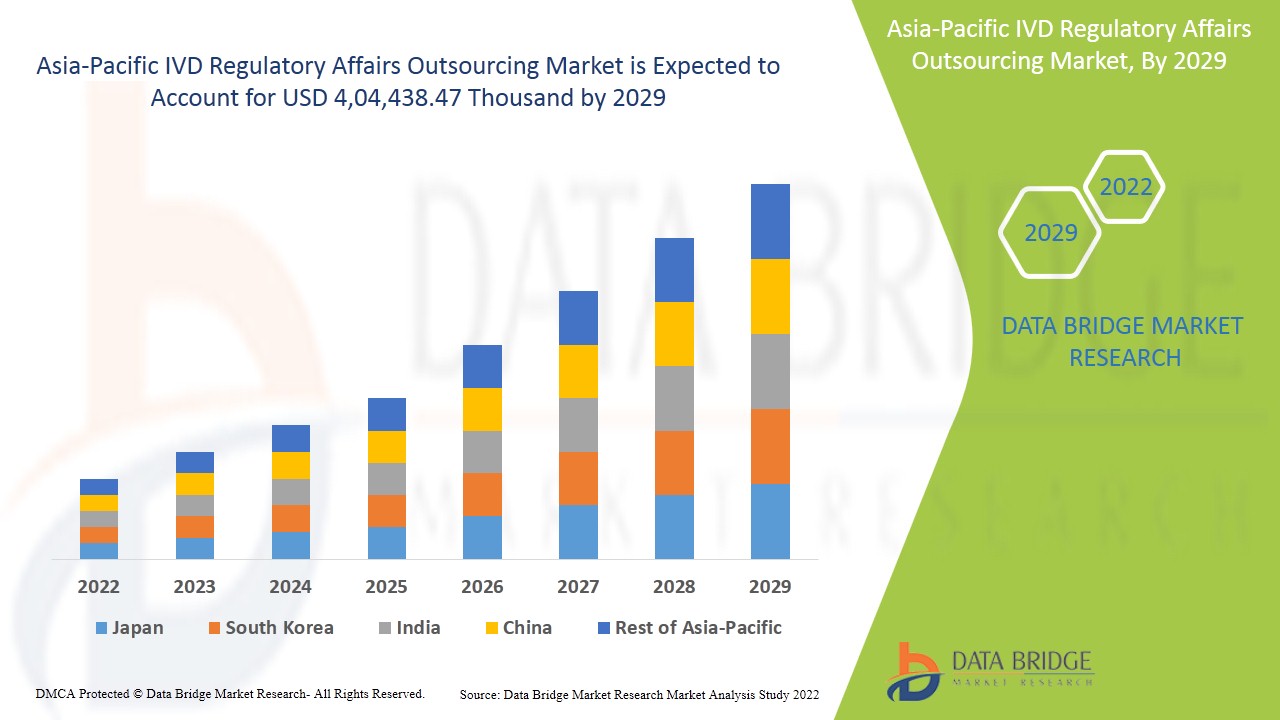
Ожидается, что рынок аутсорсинга регуляторных вопросов IVD в Азиатско-Тихоокеанском регионе будет расти в прогнозируемый период с 2022 по 2029 год. По данным Data Bridge Market Research, среднегодовой темп роста рынка составит 14,1% в прогнозируемый период с 2022 по 2029 год, а к 2029 году он, как ожидается, достигнет 4 04 438,47 тыс. долларов США.
- Продукция для диагностики in vitro — это реагенты, устройства и системы, используемые для диагностики заболеваний или других состояний, включая определение состояния здоровья для лечения, смягчения, лечения или профилактики заболеваний. Эти продукты предназначены для использования при сборе, подготовке и исследовании образцов человеческого тела. Нормативные вопросы играют важную роль в индустрии диагностических устройств in vitro (IVD) и медицинских устройств. Услуги аутсорсинга нормативно-правовых вопросов включают в себя написание и публикацию медицинской нормативной документации специалистами, которые вносят вклад в создание высококачественных документов для проектов клинических исследований. Спрос на аутсорсинг нормативно-правовых услуг существенно растет в клинических исследованиях, проводимых в странах с развивающейся экономикой, что обеспечивает здоровую платформу для роста этой отрасли.
Основными факторами, обусловливающими рост рынка аутсорсинга регуляторных вопросов IVD, являются рост распространенности хронических заболеваний в регионе и технологический прогресс в различных диагностических устройствах in vitro. Рост стратегических приобретений и партнерств между организациями создает возможности для роста рынка. Изменение правил в отношении медицинских устройств в разных регионах выступает в качестве основного ограничения для рынка аутсорсинга регуляторных вопросов IVD. Отсутствие инфраструктуры в сфере здравоохранения выступает в качестве основного препятствия для роста рынка.
В этом отчете IVD по аутсорсингу рынка регуляторных вопросов содержатся сведения о доле рынка, новых разработках и анализе продуктового портфеля, влиянии внутренних и локальных игроков рынка, анализируются возможности с точки зрения новых источников дохода, изменений в рыночных правилах, одобрений продуктов, стратегических решений, запусков продуктов, географических расширений и технологических инноваций на рынке. Чтобы понять анализ и рыночный сценарий, свяжитесь с нами для получения аналитического обзора, наша команда поможет вам создать решение по влиянию на доход для достижения желаемой цели.
Масштаб и размер рынка аутсорсинга нормативно-правового регулирования IVD в Азиатско-Тихоокеанском регионе
Рынок аутсорсинга нормативно-правового регулирования IVD в Азиатско-Тихоокеанском регионе разделен на семь основных сегментов, которые основаны на услугах, показаниях, режиме развертывания, размере организации, этапе, классе и конечном пользователе.
- На основе услуг рынок аутсорсинга регуляторных вопросов IVD в Азиатско-Тихоокеанском регионе сегментирован на написание и подачу нормативных документов, нормативную регистрацию и заявки на клинические испытания, нормативный консалтинг, юридическое представительство, услуги по управлению данными, услуги по производству и контролю химии (CMC) и другие. Ожидается, что в 2022 году на рынке будут доминировать нормативные документы и подача нормативных документов, поскольку они увеличивают количество регистраций продуктов и одобрений клинических испытаний по всему региону.
- На основе показаний рынок аутсорсинга вопросов регулирования IVD в Азиатско-Тихоокеанском регионе сегментирован на онкологию, неврологию, кардиологию, клиническую химию и иммуноанализы, точную медицину, инфекционные заболевания, диабет, генетическое тестирование, ВИЧ/СПИД, гематологию, тестирование на наркотики/фармакогеномику, переливание крови, пункты оказания медицинской помощи и др. Ожидается, что в 2022 году сегмент онкологии будет доминировать, поскольку высококачественные технологии и портфель услуг, связанных с применением регулирования IVD, привлекут большее участие коллег.
- На основе режима развертывания рынок аутсорсинга регуляторных вопросов IVD в Азиатско-Тихоокеанском регионе сегментируется на облачные и локальные. Ожидается, что в 2022 году облачный сегмент будет доминировать, поскольку он экономически эффективен, а гибкие характеристики решения технологии облачных вычислений в регулировании IVD обеспечивают устойчивую инфраструктуру с максимальной производительностью для организаций, занимающихся системами IVD.
- На основе размера организации рынок аутсорсинга регуляторных вопросов IVD в Азиатско-Тихоокеанском регионе сегментируется на малые и средние предприятия (МСП) и крупные предприятия. Ожидается, что в 2022 году сегмент крупных предприятий будет доминировать, поскольку он включает в себя юридическое представительство продукта и из-за строгих правил, которые могут различаться в зависимости от страны, предполагает высокое потребление ресурсов из-за высокой потребности в ресурсах и изменений в политике.
- На основе этапа рынок аутсорсинга регуляторных вопросов IVD в Азиатско-Тихоокеанском регионе сегментирован на клинический, доклинический и PMA (послепродажное разрешение). Ожидается, что в 2022 году клинический сегмент будет доминировать на рынке. Участники рынка устройств IVD должны соблюдать определенные правила, чтобы получить одобрение от высших органов власти для запуска продукта в регионе. Эти строгие правила необходимо соблюдать, и это одна из самых сложных задач среди всех этапов. Предпродажное одобрение различных медицинских устройств различается в разных странах.
- На основе класса рынок аутсорсинга регуляторных вопросов IVD в Азиатско-Тихоокеанском регионе сегментируется на класс I, класс II и класс III. Ожидается, что в 2022 году сегмент класса I будет доминировать на рынке, поскольку он охватывает 47% медицинских устройств и не имеет риска для общественного здоровья или имеет низкий личный риск с минимальными правилами.
- На основе конечного пользователя рынок аутсорсинга вопросов регулирования IVD в Азиатско-Тихоокеанском регионе сегментирован на фармацевтические компании, компании по производству медицинских приборов, биотехнологические компании и т. д. В 2022 году ожидается, что компании по производству медицинских приборов будут заниматься НИОКР по всему региону из-за спроса на регулирование IVD.
Анализ рынка аутсорсинга нормативно-правового регулирования IVD в Азиатско-Тихоокеанском регионе на уровне страны
Проведен анализ рынка аутсорсинга нормативно-правового регулирования IVD в Азиатско-Тихоокеанском регионе, а также предоставлена информация о размере рынка по странам, услугам, показаниям, режиму развертывания, размеру организации, этапу, классу и конечному пользователю.
В отчете о рынке аутсорсинга регулирования IVD в Азиатско-Тихоокеанском регионе рассматриваются следующие страны: Китай, Южная Корея, Япония, Индия, Австралия, Сингапур, Малайзия, Индонезия, Таиланд, Филиппины и остальные страны Азиатско-Тихоокеанского региона.
Ожидается, что Китай будет доминировать на рынке Азиатско-Тихоокеанского региона благодаря растущим разработкам в секторе здравоохранения и наличию крупнейшей клинической лаборатории в Азиатско-Тихоокеанском регионе.
Раздел отчета по странам также содержит отдельные факторы, влияющие на рынок, и изменения в регулировании на внутреннем рынке, которые влияют на текущие и будущие тенденции рынка. Такие данные, как новые продажи, замещающие продажи, демографические данные страны, нормативные акты и импортно-экспортные тарифы, являются одними из основных указателей, используемых для прогнозирования рыночного сценария для отдельных стран. Кроме того, при предоставлении прогнозного анализа данных по стране учитываются наличие и доступность глобальных брендов и их проблемы из-за большой или малой конкуренции со стороны местных и отечественных брендов, влияние каналов продаж.
Растущий спрос на аутсорсинг вопросов регулирования IVD
Азиатско-Тихоокеанский рынок аутсорсинга регуляторных вопросов IVD также предоставляет вам подробный анализ рынка для каждой страны, рост в отрасли с продажами, продажами компонентов, влиянием технологического развития на аутсорсинг регуляторных вопросов IVD и изменениями в сценариях регулирования с их поддержкой для рынка аутсорсинга регуляторных вопросов IVD. Данные доступны за исторический период с 2011 по 2019 год.
Конкурентная среда и анализ доли рынка аутсорсинга нормативно-правового регулирования IVD в Азиатско-Тихоокеанском регионе
Конкурентная среда рынка аутсорсинга регуляторных вопросов IVD в Азиатско-Тихоокеанском регионе содержит сведения по конкурентам. Включены сведения о компании, финансы компании, полученный доход, рыночный потенциал, инвестиции в исследования и разработки, новые рыночные инициативы, глобальное присутствие, производственные площадки и объекты, сильные и слабые стороны компании, запуск продукта, испытания продуктов, одобрения продуктов, патенты, ширина и широта продукта, доминирование приложений, кривая жизненной линии технологий. Приведенные выше точки данных связаны только с фокусом компаний, связанным с рынком аутсорсинга регуляторных вопросов IVD в Азиатско-Тихоокеанском регионе.
Некоторые из основных игроков, работающих в отчете, на рынке аутсорсинга регуляторных вопросов IVD: Freyr Solutions, PPD Inc. (дочерняя компания Thremofisher Scientific Inc.), EMERGO, ICON, Parexel International Corporation, CRITERIUM, INC., Groupe ProductLife SA, Labcorp Drug Development, WuXi AppTec, Genpact, Medpace, Dor Pharmaceutical Services, Qserve и другие. Аналитики DBMR понимают конкурентные преимущества и предоставляют конкурентный анализ для каждого конкурента отдельно.
Многие разработки продуктов также инициируются компаниями по всему миру, что также ускоряет рост рынка аутсорсинга регуляторных вопросов в области IVD в Азиатско-Тихоокеанском регионе.
Например,
- В ноябре 2021 года журнал USA-9 Technology Magazine включил Freyr в список «10 лучших поставщиков технологических решений 2021 года». USA-9.com — это технологический журнал, который включил Freyr Solutions, ведущего мирового поставщика решений и услуг в области регулирования, в список «10 лучших поставщиков технологических решений 2021 года», поскольку Fryer продолжает разрабатывать инновационные программные решения и оказывать поддержку клиентам в достижении их соответствующих целей по обеспечению соответствия. Это помогло компании повысить свою популярность.
- В октябре 2021 года группа Propharma приобрела Pharmica Consulting. ProPharma Group, портфельная компания Odyssey Investment Partners, приобрела Pharmica Consulting, консалтинговую компанию в области естественных наук, которая предоставляет консалтинговые решения по управлению проектами (PM) и фирменное программное обеспечение для операций фармацевтическим и биотехнологическим компаниям для проведения клинических испытаний. Это помогло компании расширить свой бизнес на глобальном рынке.
Партнерство, совместные предприятия и другие стратегии увеличивают долю рынка компании за счет увеличения охвата и присутствия. Это также дает преимущество организациям в улучшении их предложения по аутсорсингу регуляторных вопросов IVD за счет расширения диапазона размеров.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Интерактивная панель анализа данных
- Панель анализа компании для возможностей с высоким потенциалом роста
- Доступ аналитика-исследователя для настройки и запросов
- Анализ конкурентов с помощью интерактивной панели
- Последние новости, обновления и анализ тенденций
- Используйте возможности сравнительного анализа для комплексного отслеживания конкурентов
Содержание
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 DBMR MARKET POSITION GRID
2.7 VENDOR SHARE ANALYSIS
2.8 MULTIVARIATE MODELING
2.9 SERVICE TIMELINE CURVE
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, REGULATORY SCENARIO
4.1.1 THE U.S.
4.1.2 REGULATIONS IN EUROPE
4.1.3 REGULATIONS IN ASIA
4.1.3.1 CHINA
4.1.3.2 SOUTH KOREA
4.1.3.3 MALAYSIA
4.1.3.4 THAILAND
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISE IN PREVALENCE OF CHRONIC DISEASES ACROSS THE REGION
5.1.2 TECHNOLOGICAL ADVANCEMENT IN DEVELOPING VARIOUS IN VITRO DIAGNOSTIC DEVICES
5.1.3 DEVELOPMENT OF PROJECT-BASED SUPPORT LEADS TO LONG TERM OUTSOURCING AGREEMENT AMONG ORGANIZATION
5.1.4 INCREASE IN PRODUCT REGISTRATION NUMBERS AND CLINICAL TRIAL APPROVALS ACROSS THE REGION
5.2 RESTRAINTS
5.2.1 STRINGENT REGULATIONS REGARDING MEDICAL DEVICES IN DIFFERENT REGIONS
5.2.2 HIGHER COST RELATED TO MAINTENANCE AND OUTSOURCING OF IVD
5.3 OPPORTUNITIES
5.3.1 RISE IN STRATEGIC ACQUISITION & PARTNERSHIP AMONG ORGANIZATION
5.3.2 EMERGENCE OF VARIOUS EFFICIENT TECHNOLOGICAL SERVICES AND STANDARDS
5.3.3 INCREASE IN R&D ACTIVITIES BY COMPANIES ACROSS THE REGION
5.4 CHALLENGES
5.4.1 LACK OF INFRASTRUCTURE IN HEALTHCARE SERVICE
5.4.2 SHORTAGE OF SKILLED PERSONNEL FOR HANDLING IN VITRO DIAGNOSTIC DEVICES
6 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE
6.1 OVERVIEW
6.2 REGULATORY WRITING & SUBMISSIONS
6.3 LEGAL REPRESENTATION
6.4 REGULATORY CONSULTING
6.5 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
6.6 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
6.7 DATA MANAGEMENT SERVICES
6.8 OTHERS
7 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION
7.1 OVERVIEW
7.2 CLINICAL CHEMISTRY AND IMMUNOASSAYS
7.3 INFECTIOUS DISEASES
7.3.1 VIROLOGY
7.3.2 MICROBIOLOGY AND MYCOLOGY
7.3.3 BACTERIOLOGY
7.3.4 SEPSIS
7.3.5 HEPATITIS B
7.3.6 HEPATITIS C
7.3.7 MALARIA
7.3.8 TUBERCULOSIS
7.3.9 SYPHILIS
7.3.10 HUMAN PAPILLOMAVIRUS (HPV) INFECTION
7.3.11 OTHERS
7.4 HAEMATOLOGY
7.5 DRUG TESTING/PHARMACOGENOMICS
7.6 PRECISION MEDICINE
7.7 DIABETES
7.8 BLOOD TRANSFUSION
7.9 CARDIOLOGY
7.1 POINT OF CARE
7.10.1 WAIVED TEST
7.10.2 AT HOME TESTS
7.11 ONCOLOGY
7.12 NEUROLOGY
7.13 HIV/AIDS
7.14 GENETIC TESTING
7.15 OTHERS
8 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS
8.1 OVERVIEW
8.2 CLASS I
8.3 CLASS III
8.4 CLASS II
9 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE
9.1 OVERVIEW
9.2 CLOUD
9.3 ON-PREMISES
10 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE
10.1 OVERVIEW
10.2 LARGE ENTERPRISES
10.3 SMALL & MEDIUM ENTERPRISES (SMES)
11 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE
11.1 OVERVIEW
11.2 CLINICAL
11.3 PRECLINICAL
11.4 PMA (POST MARKET AUTHORIZATION)
12 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER
12.1 OVERVIEW
12.2 MEDICAL DEVICE COMPANIES
12.2.1 BY ORGANIZATION SIZE
12.2.1.1 LARGE ENTERPRISES
12.2.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.2.2 BY SERVICE
12.2.2.1 REGULATORY WRITING & SUBMISSIONS
12.2.2.2 LEGAL REPRESENTATION
12.2.2.3 REGULATORY CONSULTING
12.2.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.2.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.2.2.6 DATA MANAGEMENT SERVICES
12.2.2.7 OTHERS
12.3 PHARMACEUTICAL COMPANIES
12.3.1 BY ORGANIZATION SIZE
12.3.1.1 LARGE ENTERPRISES
12.3.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.3.2 BY SERVICE
12.3.2.1 REGULATORY WRITING & SUBMISSIONS
12.3.2.2 LEGAL REPRESENTATION
12.3.2.3 REGULATORY CONSULTING
12.3.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.3.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.3.2.6 DATA MANAGEMENT SERVICES
12.3.2.7 OTHERS
12.4 BIOTECHNOLOGY COMPANIES
12.4.1 BY ORGANIZATION SIZE
12.4.1.1 LARGE ENTERPRISES
12.4.1.2 SMALL & MEDIUM ENTERPRISES (SMES)
12.4.2 BY SERVICE
12.4.2.1 REGULATORY WRITING & SUBMISSIONS
12.4.2.2 LEGAL REPRESENTATION
12.4.2.3 REGULATORY CONSULTING
12.4.2.4 REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS
12.4.2.5 CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES
12.4.2.6 DATA MANAGEMENT SERVICES
12.4.2.7 OTHERS
12.5 OTHERS
13 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 JAPAN
13.1.3 INDIA
13.1.4 SOUTH KOREA
13.1.5 AUSTRALIA
13.1.6 SINGAPORE
13.1.7 THAILAND
13.1.8 MALAYSIA
13.1.9 INDONESIA
13.1.10 PHILIPPINES
13.1.11 REST OF ASIA-PACIFIC
14 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ICON PLC
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 MEDPACE
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 PAREXEL INTERNATIONAL CORPORATION
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENTS
16.4 LABCORP DRUG DEVELOPMENT
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENT
16.5 PPD INC. (A SUBSIDIARY OF THERMOFISHER SCIENTIFIC INC.)
16.5.1 COMPANY SNAPSHOT
16.5.2 COMPANY SHARE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 CHARLES RIVER LABORATORIES
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 FREYR
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 ASSENT COMPLIANCE INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 ANDAMAN MEDICAL
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 ASIA ACTUAL
16.10.1 COMPANY SNAPSHOT
16.10.2 SERVICE PORTFOLIO
16.10.3 RECENT DEVELOPMENTS
16.11 AXSOURCE
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 CRITERIUM, INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 DOR PHARMACEUTICAL SERVICES
16.13.1 COMPANY SNAPSHOT
16.13.2 SERVICE PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 EMERGO BY UL
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 GENPACT
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GROUPE PRODUCTLIFE S.A.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 LORENZ LIFE SCIENCES GROUP
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 MAKROCARE
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 MARACA INTERNATIONAL BVBA
16.19.1 COMPANY SNAPSHOT
16.19.2 SERVICE PORTFOLIO
16.19.3 RECENT DEVELOPMENTS
16.2 MDICONSULTANTS, INC.
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 PBC BIOMED
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 PROMEDICA INTERNATIONAL, A CALIFORNIA CORPORATION
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENT
16.23 PROPHARMA GROUP
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENTS
16.24 QSERVE
16.24.1 COMPANY SNAPSHOT
16.24.2 SERVICE PORTFOLIO
16.24.3 RECENT DEVELOPMENTS
16.25 REGULATORY COMPLIANCE ASSOCIATES INC.
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENT
16.26 RMQ+
16.26.1 COMPANY SNAPSHOT
16.26.2 PRODUCT PORTFOLIO
16.26.3 RECENT DEVELOPMENT
16.27 SARACA SOLUTIONS PRIVATE LIMITED
16.27.1 COMPANY SNAPSHOT
16.27.2 PRODUCT PORTFOLIO
16.27.3 RECENT DEVELOPMENT
16.28 VCLS
16.28.1 COMPANY SNAPSHOT
16.28.2 PRODUCT PORTFOLIO
16.28.3 RECENT DEVELOPMENTS
16.29 WUXI APPTEC
16.29.1 COMPANY SNAPSHOT
16.29.2 REVENUE ANALYSIS
16.29.3 PRODUCT PORTFOLIO
16.29.4 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
Список таблиц
TABLE 1 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 2 ASIA PACIFIC REGULATORY WRITING & SUBMISSIONS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 3 ASIA PACIFIC LEGAL REPRESENTATION IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 4 ASIA PACIFIC REGULATORY CONSULTING IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 5 ASIA PACIFIC REGULATORY REGISTRATION & CLINICAL TRIAL APPLICATIONS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 6 ASIA PACIFIC CHEMISTRY MANUFACTURING AND CONTROLS (CMC) SERVICES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 7 ASIA PACIFIC DATA MANAGEMENT SERVICES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY MATERIAL, 2020-2029 (USD THOUSAND)
TABLE 8 ASIA PACIFIC OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 9 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 10 ASIA PACIFIC CLINICAL CHEMISTRY AND IMMUNOASSAYS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 11 ASIA PACIFIC INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION,2020-2029 (THOUSAND)
TABLE 12 ASIA PACIFIC INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 13 ASIA PACIFIC HAEMATOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 14 ASIA PACIFIC DRUG TESTING/PHARMACOGENOMICS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 15 ASIA PACIFIC PRECISION MEDICINE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 16 ASIA PACIFIC DIABETES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 17 ASIA PACIFIC BLOOD TRANSFUSION IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 18 ASIA PACIFIC CARDIOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 19 ASIA PACIFIC POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 20 ASIA PACIFIC POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 21 ASIA PACIFIC ONCOLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 22 ASIA PACIFIC NEUROLOGY IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 23 ASIA PACIFIC HIV/AIDS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 24 ASIA PACIFIC GENETIC TESTING IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 25 ASIA PACIFIC OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 26 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 27 ASIA PACIFIC CLASS I IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 28 ASIA PACIFIC CLASS III IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (THOUSAND)
TABLE 29 ASIA PACIFIC CLASS II IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 30 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 31 ASIA PACIFIC CLOUD IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 32 ASIA PACIFIC ON-PREMISES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 33 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 34 ASIA PACIFIC LARGE ENTERPRISES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 35 ASIA PACIFIC SMALL & MEDIUM ENTERPRISES (SMES) IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 36 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 37 ASIA PACIFIC CLINICAL IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 38 ASIA PACIFIC PRECLINICAL IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 39 ASIA PACIFIC PMA (POST MARKET AUTHORIZATION) IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 40 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 41 ASIA PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 42 ASIA PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 43 ASIA PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 44 ASIA PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 45 ASIA PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 46 ASIA PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 47 ASIA PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 48 ASIA PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 49 ASIA PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 50 ASIA PACIFIC OTHERS IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2020-2029 (USD THOUSAND)
TABLE 51 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY COUNTRY, 2020-2029 (USD THOUSAND)
TABLE 52 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 53 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 54 ASIA-PACIFIC INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 55 ASIA-PACIFIC POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 56 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 57 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 58 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 59 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 60 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 61 ASIA-PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 62 ASIA-PACIFIC MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 63 ASIA-PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 64 ASIA-PACIFIC PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 65 ASIA-PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 66 ASIA-PACIFIC BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 67 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 68 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 69 CHINA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 70 CHINA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 71 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 72 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 73 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 74 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 75 CHINA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 76 CHINA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 77 CHINA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 78 CHINA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 79 CHINA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 80 CHINA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 81 CHINA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 82 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 83 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 84 JAPAN INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 85 JAPAN POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 86 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 87 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 88 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 89 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 90 JAPAN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 91 JAPAN MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 92 JAPAN MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 93 JAPAN PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 94 JAPAN PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 95 JAPAN BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 96 JAPAN BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 97 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 98 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 99 INDIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 100 INDIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 101 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 102 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 103 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 104 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 105 INDIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 106 INDIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 107 INDIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 108 INDIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 109 INDIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 110 INDIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 111 INDIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 112 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 113 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 114 SOUTH KOREA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 115 SOUTH KOREA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 116 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 117 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 118 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 119 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 120 SOUTH KOREA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 121 SOUTH KOREA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 122 SOUTH KOREA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 123 SOUTH KOREA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 124 SOUTH KOREA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 125 SOUTH KOREA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 126 SOUTH KOREA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 127 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 128 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 129 AUSTRALIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 130 AUSTRALIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 131 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 132 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 133 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 134 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 135 AUSTRALIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 136 AUSTRALIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 137 AUSTRALIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 138 AUSTRALIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 139 AUSTRALIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 140 AUSTRALIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 141 AUSTRALIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 142 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 143 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 144 SINGAPORE INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 145 SINGAPORE POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 146 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 147 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 148 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 149 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 150 SINGAPORE IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 151 SINGAPORE MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 152 SINGAPORE MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 153 SINGAPORE PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 154 SINGAPORE PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 155 SINGAPORE BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 156 SINGAPORE BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 157 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 158 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 159 THAILAND INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 160 THAILAND POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 161 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 162 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 163 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 164 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 165 THAILAND IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 166 THAILAND MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 167 THAILAND MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 168 THAILAND PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 169 THAILAND PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 170 THAILAND BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 171 THAILAND BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 172 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 173 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 174 MALAYSIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 175 MALAYSIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 176 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 177 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 178 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 179 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 180 MALAYSIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 181 MALAYSIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 182 MALAYSIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 183 MALAYSIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 184 MALAYSIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 185 MALAYSIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 186 MALAYSIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 187 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 188 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 189 INDONESIA INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 190 INDONESIA POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 191 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 192 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 193 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 194 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 195 INDONESIA IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 196 INDONESIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 197 INDONESIA MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 198 INDONESIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 199 INDONESIA PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 200 INDONESIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 201 INDONESIA BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 202 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 203 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY INDICATION, 2020-2029 (USD THOUSAND)
TABLE 204 PHILIPPINES INFECTIOUS DISEASES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 205 PHILIPPINES POINT OF CARE IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY TYPE, 2020-2029 (USD THOUSAND)
TABLE 206 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEPLOYMENT MODE, 2020-2029 (USD THOUSAND)
TABLE 207 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 208 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY STAGE, 2020-2029 (USD THOUSAND)
TABLE 209 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY CLASS, 2020-2029 (USD THOUSAND)
TABLE 210 PHILIPPINES IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER, 2020-2029 (USD THOUSAND)
TABLE 211 PHILIPPINES MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 212 PHILIPPINES MEDICAL DEVICE COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 213 PHILIPPINES PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 214 PHILIPPINES PHARMACEUTICAL COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 215 PHILIPPINES BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY ORGANIZATION SIZE, 2020-2029 (USD THOUSAND)
TABLE 216 PHILIPPINES BIOTECHNOLOGY COMPANIES IN IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
TABLE 217 REST OF ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICE, 2020-2029 (USD THOUSAND)
Список рисунков
FIGURE 1 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: END USER COVERAGE GRID
FIGURE 10 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SEGMENTATION
FIGURE 11 RISE IN THE PREVALENCE OF CHRONIC DISEASES ACROSS IS EXPECTED TO DRIVE ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SERVICE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN 2022 & 2029
FIGURE 13 ASIA-PACIFIC IS EXPECTED TO DOMINATE AND IS THE FASTEST GROWING REGION IN THE ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES FOR ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET
FIGURE 15 MULTIPLE CHRONIC CONDITIONS AMONG PEOPLE AGED 65 AND ABOVE IN EUROPEAN REGION (2017)
FIGURE 16 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY SERVICE, 2021
FIGURE 17 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY INDICATION, 2021
FIGURE 18 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY CLASS, 2021
FIGURE 19 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY DEPLOYMENT MODE, 2021
FIGURE 20 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY ORGANIZATION SIZE, 2021
FIGURE 21 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY STAGE, 2021
FIGURE 22 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY END USER, 2021
FIGURE 23 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: SNAPSHOT (2021)
FIGURE 24 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2021)
FIGURE 25 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2022 & 2029)
FIGURE 26 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY COUNTRY (2021 & 2029)
FIGURE 27 ASIA-PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: BY SERVICE (2022-2029)
FIGURE 28 ASIA PACIFIC IVD REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY SHARE 2021 (%)

Методология исследования
Сбор данных и анализ базового года выполняются с использованием модулей сбора данных с большими размерами выборки. Этап включает получение рыночной информации или связанных данных из различных источников и стратегий. Он включает изучение и планирование всех данных, полученных из прошлого заранее. Он также охватывает изучение несоответствий информации, наблюдаемых в различных источниках информации. Рыночные данные анализируются и оцениваются с использованием статистических и последовательных моделей рынка. Кроме того, анализ доли рынка и анализ ключевых тенденций являются основными факторами успеха в отчете о рынке. Чтобы узнать больше, пожалуйста, запросите звонок аналитика или оставьте свой запрос.
Ключевой методологией исследования, используемой исследовательской группой DBMR, является триангуляция данных, которая включает в себя интеллектуальный анализ данных, анализ влияния переменных данных на рынок и первичную (отраслевую экспертную) проверку. Модели данных включают сетку позиционирования поставщиков, анализ временной линии рынка, обзор рынка и руководство, сетку позиционирования компании, патентный анализ, анализ цен, анализ доли рынка компании, стандарты измерения, глобальный и региональный анализ и анализ доли поставщика. Чтобы узнать больше о методологии исследования, отправьте запрос, чтобы поговорить с нашими отраслевыми экспертами.
Доступна настройка
Data Bridge Market Research является лидером в области передовых формативных исследований. Мы гордимся тем, что предоставляем нашим существующим и новым клиентам данные и анализ, которые соответствуют и подходят их целям. Отчет можно настроить, включив в него анализ ценовых тенденций целевых брендов, понимание рынка для дополнительных стран (запросите список стран), данные о результатах клинических испытаний, обзор литературы, обновленный анализ рынка и продуктовой базы. Анализ рынка целевых конкурентов можно проанализировать от анализа на основе технологий до стратегий портфеля рынка. Мы можем добавить столько конкурентов, о которых вам нужны данные в нужном вам формате и стиле данных. Наша команда аналитиков также может предоставить вам данные в сырых файлах Excel, сводных таблицах (книга фактов) или помочь вам в создании презентаций из наборов данных, доступных в отчете.