Растущая распространенность хронических заболеваний, таких как диабет, сердечно-сосудистые заболевания и инфекционные заболевания, стала важнейшим фактором для рынка наборов для домашнего тестирования в Латинской Америке. Эти хронические состояния требуют постоянного мониторинга показателей здоровья, включая уровень глюкозы в крови, холестерина и артериального давления, для обеспечения эффективного лечения и предотвращения осложнений. Традиционно пациентам приходилось часто посещать медицинские учреждения для таких тестов, но растущий спрос на удобство привел к переходу к домашним диагностическим решениям. Наборы для домашнего тестирования позволяют людям регулярно контролировать свое здоровье, не выходя из дома, что особенно полезно для тех, у кого ограничен доступ к медицинским учреждениям или в сельской местности.
Необходимость раннего выявления также стимулирует этот спрос. Хронические заболевания часто прогрессируют постепенно, и раннее выявление аномальных показателей здоровья может иметь существенное значение для предотвращения ухудшения состояния. Наборы для домашнего тестирования дают пациентам возможность проводить регулярные самопроверки, помогая им выявлять предупреждающие признаки на ранней стадии и своевременно обращаться за медицинской помощью. Кроме того, удобство домашнего тестирования сокращает время, усилия и расходы, связанные с частыми визитами к врачу, что является привлекательным фактором для пациентов, имеющих длительные заболевания.
Доступ к полному отчету по адресу https://www.databridgemarketresearch.com/reports/latin-america-at-home-testing-kits-market
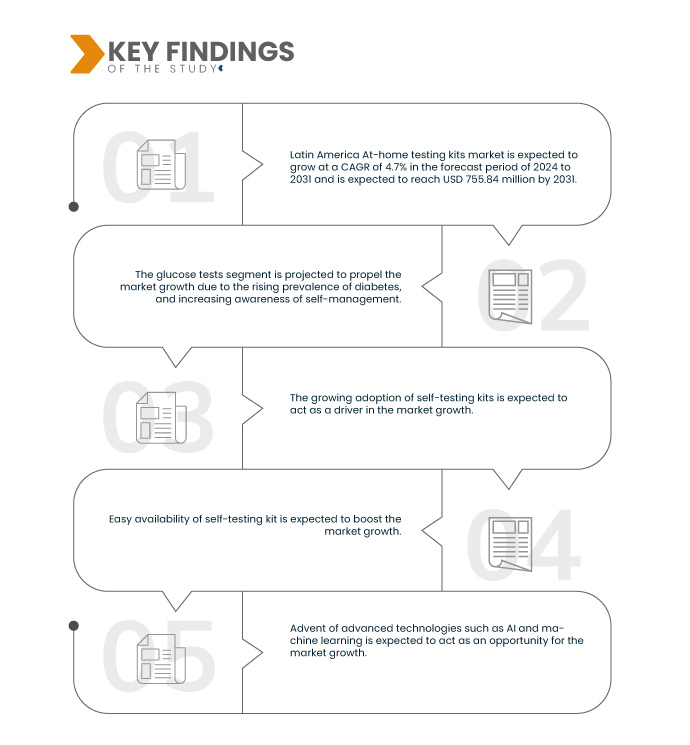
По данным исследования рынка Data Bridge, ожидается, что среднегодовой темп роста рынка наборов для домашнего тестирования в Латинской Америке составит 4,7% в прогнозируемый период с 2024 по 2031 год, а объем рынка достигнет 755,84 млн долларов США к 2031 году по сравнению с 525,88 млн долларов США в 2023 году.
Основные выводы исследования
«Растущее использование наборов для самотестирования»
Раньше люди часто посещали больницы даже по основным медицинским проблемам. Однако с ростом осведомленности о продуктах для домашнего тестирования это поведение значительно изменилось. Сегодня многие потребители предпочитают использовать наборы для самостоятельного тестирования дома для основных тестов перед тем, как обратиться к врачу. Эта растущая тенденция обеспечивает удобство, поскольку люди могут быстро и легко получить доступ к результатам своих тестов. В Латинской Америке растущее внедрение наборов для самостоятельного тестирования является ключевым фактором рынка наборов для домашнего тестирования, особенно в регионах с ограниченной инфраструктурой здравоохранения. Эти наборы предоставляют пациентам доступное, надежное и удобное решение для контроля за своим здоровьем, снижая необходимость частых посещений больницы. Они особенно полезны в сельских и недостаточно обслуживаемых районах, позволяя на ранней стадии выявлять и лечить такие состояния, как диабет, COVID-19 и инфекции, передающиеся половым путем. Уменьшая переполненность больниц и снижая плату за консультации и диагностику, наборы для самостоятельного тестирования не только улучшают доступ к здравоохранению, но и помогают сократить расходы на больницы, что делает их практичным решением как для пациентов, так и для систем здравоохранения.
Растущее распространение наборов для самостоятельного тестирования в Латинской Америке стимулирует рынок наборов для домашнего тестирования, решая проблемы доступности здравоохранения и значительно сокращая расходы больниц, предлагая практичное решение как для пациентов, так и для систем здравоохранения.
Область отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2024-2031
|
Базовый год
|
2023
|
Исторический год
|
2022 (можно изменить на 2016–2021)
|
Количественные единицы
|
Доход в млн. долл. США и цены в долл. США
|
Охваченные сегменты
|
Тип теста (тест на беременность, инфекционные заболевания , тесты на глюкозу, набор для определения овуляции, наборы для определения злоупотребления наркотиками и другие типы тестов), тип (кассеты, полоски, мидстрим, тестовая панель, погружная карта и другие), возраст (детский, взрослый и гериатрический), тип образца (моча, кровь, слюна и другие типы образцов), использование (одноразовые и многоразовые), канал распространения (розничные аптеки, аптека, супермаркет/гипермаркет и интернет-аптеки)
|
Страны, охваченные
|
Бразилия, Аргентина, остальные страны Латинской Америки
|
Охваченные участники рынка
|
Abbott (США), F. Hoffmann-La Roche Ltd. (Швейцария), BD (США), Siemens Healthineers AG (Германия), QuidelOrtho Corporation (США), ACON Laboratories, Inc. (США), ARKRAY, Inc. (Япония), B. Braun SE (Германия), Bionime Corporation (Тайвань), OraSure Technologies Inc. (США), SA Scientific Ltd. (США) и PRIMA Lab SA (Швейцария) и другие.
|
Данные, отраженные в отчете
|
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента
Рынок наборов для домашнего тестирования в Латинской Америке сегментирован на шесть основных сегментов в зависимости от типа теста, возраста, типа образца, использования и каналов распространения.
- По типу теста рынок сегментирован на тесты на беременность, тесты на ВИЧ, диабет, инфекционные заболевания, тесты на глюкозу, тесты для прогнозирования овуляции, тесты на злоупотребление наркотиками и другие типы тестов.
Ожидается, что в 2024 году сегмент тестов на уровень глюкозы будет доминировать на рынке.
Ожидается, что в 2025 году сегмент тестов на уровень глюкозы будет доминировать на рынке с долей рынка 51,02% благодаря растущей осведомленности о ранней диагностике, а также доступности и доступности домашних наборов.
- По типу рынок сегментируется на кассетные, полосовые, мидстримовые, тестовые панели, погружные карты и другие типы форм.
Ожидается, что в 2024 году сегмент полосового проката будет доминировать на рынке.
Ожидается, что в 2024 году сегмент полосок будет доминировать на рынке с долей рынка 52,37% из-за простоты использования, низкой стоимости и быстрого результата. Ожидается, что этот сегмент будет доминировать на рынке.
- По возрасту рынок сегментирован на педиатрический, взрослый и гериатрический. Ожидается, что в 2024 году сегмент взрослых будет доминировать на рынке с долей рынка 60,54%
- На основе типа образца рынок сегментируется на мочу, кровь, слюну и другие типы образцов. Ожидается, что в 2024 году сегмент крови будет доминировать на рынке с долей рынка 54,74%
- По принципу использования рынок сегментируется на одноразовый и многоразовый. Ожидается, что в 2024 году сегмент одноразового использования будет доминировать на рынке с долей рынка 67,45%.
- На основе канала дистрибуции рынок сегментирован на розничные аптеки, аптеки, супермаркеты/гипермаркеты и интернет-аптеки. Ожидается, что в 2024 году сегмент розничных аптек будет доминировать на рынке с долей рынка 51,63%
Основные игроки
Компания Data Bridge Market Research выделяет следующие компании в качестве основных игроков на рынке наборов для домашнего тестирования, в том числе Abbott (США), F. Hoffmann-La Roche Ltd. (Швейцария), BD (США), Siemens Healthineers AG (Германия), QuidelOrtho Corporation (США) и другие.
Развитие рынка
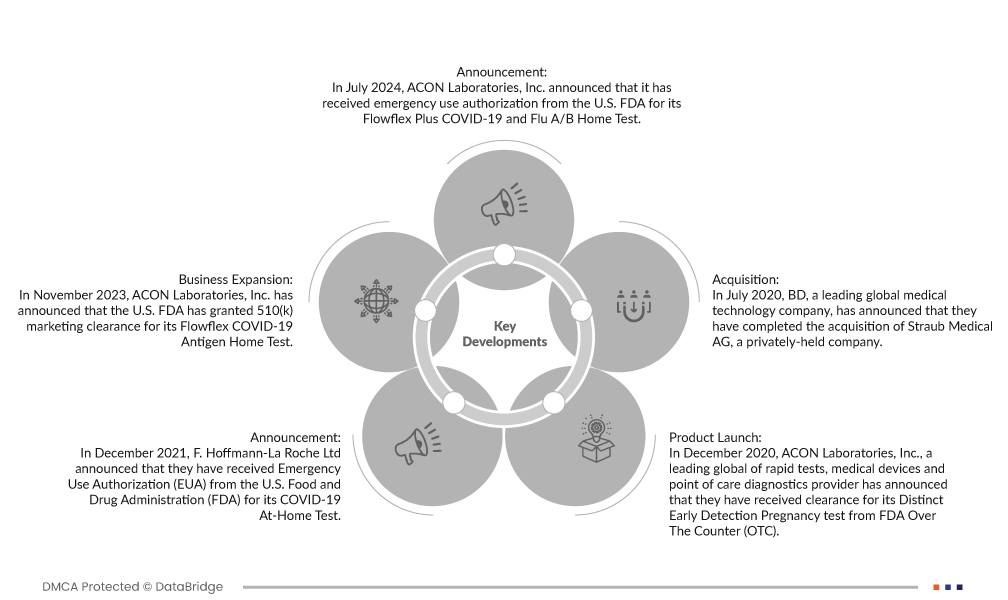
- В июле 2024 года компания ACON Laboratories, Inc. объявила о получении экстренного разрешения от FDA США на использование домашнего теста Flowflex Plus COVID-19 и гриппа A/B. Этот тест обнаруживает антигены SARS-CoV-2, гриппа A и B с использованием самостоятельно собранных мазков из носа у лиц в возрасте 14 лет и старше или образцов, собранных взрослыми, у лиц в возрасте от 2 лет и старше.
Это разрешение расширяет линейку продукции ACON и присутствие на рынке домашнего тестирования, позволяя компании удовлетворять растущий спрос и захватывать большую долю рынка тестирования в местах оказания медицинской помощи.
- В ноябре 2023 года компания ACON Laboratories, Inc. объявила, что FDA США выдало разрешение на продажу 510(k) для своего домашнего теста на антиген Flowflex COVID-19. Это первое разрешение FDA 510(k) для безрецептурного экспресс-теста на антиген SARS-CoV-2.
Это достижение выводит ACON на лидирующие позиции на рынке безрецептурных экспресс-тестов, укрепляя ее конкурентные преимущества и расширяя ассортимент продукции, что в конечном итоге способствует росту потребительского спроса на доступные решения для тестирования на COVID-19.
- В декабре 2021 года компания F. Hoffmann-La Roche Ltd объявила о получении разрешения на экстренное использование (EUA) от Управления по контролю за продуктами и лекарствами США (FDA) для своего домашнего теста на COVID-19. В этом тесте используется простой в использовании мазок из передней носовой полости, который могут самостоятельно взять лица в возрасте от 14 лет и старше, а также взрослые для детей в возрасте от 2 до 13 лет.
Это разрешение поможет компании расширить ассортимент своей продукции на рынке домашнего тестирования, укрепив ее репутацию и потенциал продаж на фоне постоянного спроса на доступные решения для тестирования на COVID-19.
- В апреле 2021 года компания Abbott объявила, что тест BinaxNOW COVID-19 Self Test теперь можно приобрести без рецепта в аптеках CVS, Walgreens и Walmart по всей стране, что делает быстрое самостоятельное тестирование на COVID-19 более доступным для населения.
- В августе 2020 года компания Siemens Healthcare GmbH заключила соглашение с Varian Medical Systems, Inc. Благодаря этому приобретению компания Siemens Healthcare будет способствовать разработке передовых решений для лечения рака и укреплению своих позиций в отрасли здравоохранения.
- В июле 2020 года BD, ведущая мировая компания в области медицинских технологий, объявила о завершении приобретения частной компании Straub Medical AG. Благодаря этому приобретению компания добавит ценные знания и опыт Straub Medical AG и расширит свой продуктовый портфель.
Региональный анализ
Географически отчет о рынке наборов для домашнего тестирования в Латинской Америке охватывает следующие страны: Бразилию, Аргентину и остальные страны Латинской Америки.
Согласно анализу Data Bridge Market Research:
Мексика будет доминирующей страной на рынке наборов для домашнего тестирования в Латинской Америке в прогнозируемый период с 2024 по 2031 год.
Ожидается, что Мексика будет доминировать на рынке благодаря своей хорошо налаженной инфраструктуре здравоохранения и высокому уровню внедрения передовых технологий, включая облачные решения и автоматизацию. Поддерживающие правительственные инициативы и значительные инвестиции в ИТ-системы здравоохранения стимулируют инновации и повышают эффективность лабораторных операций в регионе.
Более подробную информацию об отчете о рынке наборов для домашнего тестирования в Латинской Америке можно получить здесь – https://www.databridgemarketresearch.com/reports/latin-america-at-home-testing-kits-market