The breast reconstruction market incorporates various technologies, with different surgical approaches being key determinants. Inframammary reconstruction involves incisions in the breast fold, providing direct access for implant placement. Peri-areolar reconstruction utilizes incisions around the areola. Trans-axillary reconstruction accesses the breast through an incision in the armpit, while transumbilical reconstruction involves incisions near the navel. Each technology caters to diverse patient preferences and clinical considerations, offering options for breast cancer survivors seeking reconstructive surgery tailored to their specific needs and desired outcomes.
Access Full Report @ https://www.databridgemarketresearch.com/reports/europe-breast-reconstruction-market
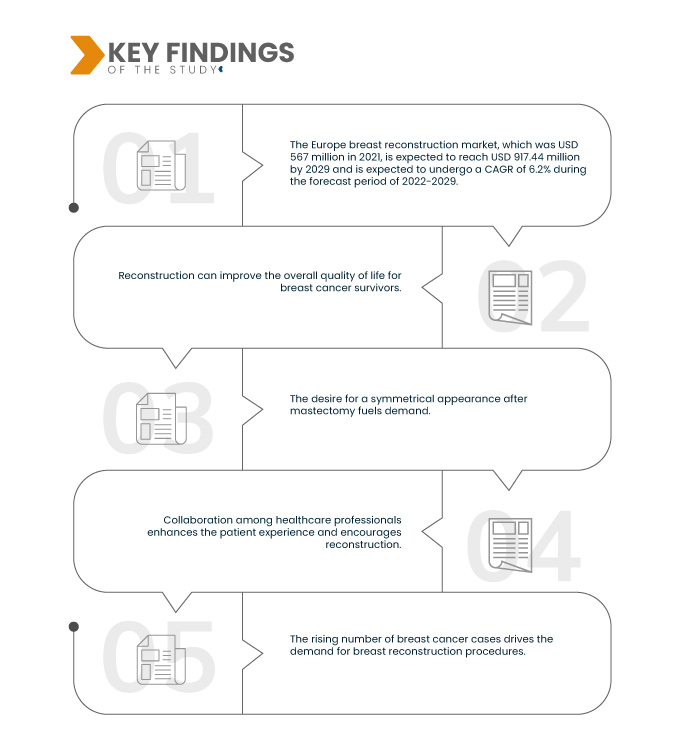
Data Bridge Market Research analyses that the Europe Breast Reconstruction Market, which was USD 567 million in 2021, is expected to reach USD 917.44 million by 2029 and is expected to undergo a CAGR of 6.2% during the forecast period of 2022-2029. The expansion of health insurance coverage for breast reconstruction procedures increases accessibility to these surgeries. It eliminates financial barriers and enables a broader range of breast cancer survivors to opt for reconstruction, enhancing their quality of life and recovery process.
Key Findings of the Study
Patient advocacy is expected to drive the market's growth rate
Patient advocacy groups play a vital role in the breast reconstruction market by providing support, information, and resources to individuals who have undergone mastectomies due to breast cancer. These groups empower patients to explore their options for breast reconstruction, ensuring they are well-informed about the available procedures, benefits, and the importance of their choices. By offering a sense of community, emotional support, and practical guidance, advocacy groups help individuals make informed decisions regarding their breast reconstruction, ultimately improving their quality of life.
Report Scope and Market Segmentation
Report Metric
|
Details
|
Forecast Period
|
2022 to 2029
|
Base Year
|
2021
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
Segments Covered
|
Product (Breast Implants, Tissue Expanders, Acellular Dermal Matrix), Type (Unilateral, Bilateral), Shape (Round Implants, Anatomical Implants), Technology (Inframammary, Peri-Areolar, Trans-Axillary, Transumbilical), Placement (Dual-Plane Insertion, Subglandular Insertion, Submuscular Insertion), Procedure (Immediate Procedures, Delayed Procedures, Revision Procedures), End User (Hospitals, Cosmetology Clinics, Ambulatory Surgical Centers, Others)
|
Countries Covered
|
Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, остальные страны Европы в Европе.
|
Охваченные участники рынка
|
POLYTECH Health & Aesthetics GmbH (Германия), CEREPLAS (Франция), Guangzhou Wanhe Plastic Material Co. Ltd (Китай), ALLERGAN (Ирландия), Johnson & Johnson Private Limited (США), GC Aesthetics (Великобритания), Laboratories Arion (Франция), Sientra Inc. (США), HANSBIOMED CO. LTD (Южная Корея), Sientra, Inc. (США), Deal Implant Incorporated (США), Integra LifeSciences (США), PMT Corporation (США), RTI Surgical (США), Establishment Labs SA (США), Silimed (Бразилия)
|
Данные, отраженные в отчете
|
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента:
Европейский рынок реконструкции груди сегментирован по продукту, типу, форме, технологии, месту установки, процедуре и конечному пользователю.
- По видам продукции европейский рынок реконструкции груди сегментирован на грудные имплантаты, тканевые экспандеры и бесклеточный дермальный матрикс.
- По типу рынок реконструкции груди в Европе сегментируется на одностороннюю и двустороннюю.
- По форме европейский рынок реконструкции груди сегментирован на круглые и анатомические имплантаты.
- По технологическому признаку европейский рынок реконструкции груди сегментирован на инфрамаммарный, периареолярный, трансаксиллярный и трансумбиликальный.
- По принципу размещения европейский рынок реконструкции груди сегментирован на двухплоскостное введение, субгландулярное введение и субмускулярное введение.
- По принципу проведения процедуры европейский рынок реконструкции груди подразделяется на немедленные процедуры, отсроченные процедуры и повторные процедуры.
- По типу конечного потребителя европейский рынок реконструкции груди сегментирован на больницы, косметологические клиники, амбулаторные хирургические центры и другие.
Основные игроки
Компания Data Bridge Market Research выделяет следующие компании в качестве игроков на европейском рынке реконструкции груди: POLYTECH Health & Aesthetics GmbH (Германия), CEREPLAS (Франция), Guangzhou Wanhe Plastic Material Co. Ltd (Китай), ALLERGAN (Ирландия), Johnson & Johnson Private Limited (США), GC Aesthetics (Великобритания), Laboratories Arion (Франция), Sientra Inc. (США).
Развитие рынка
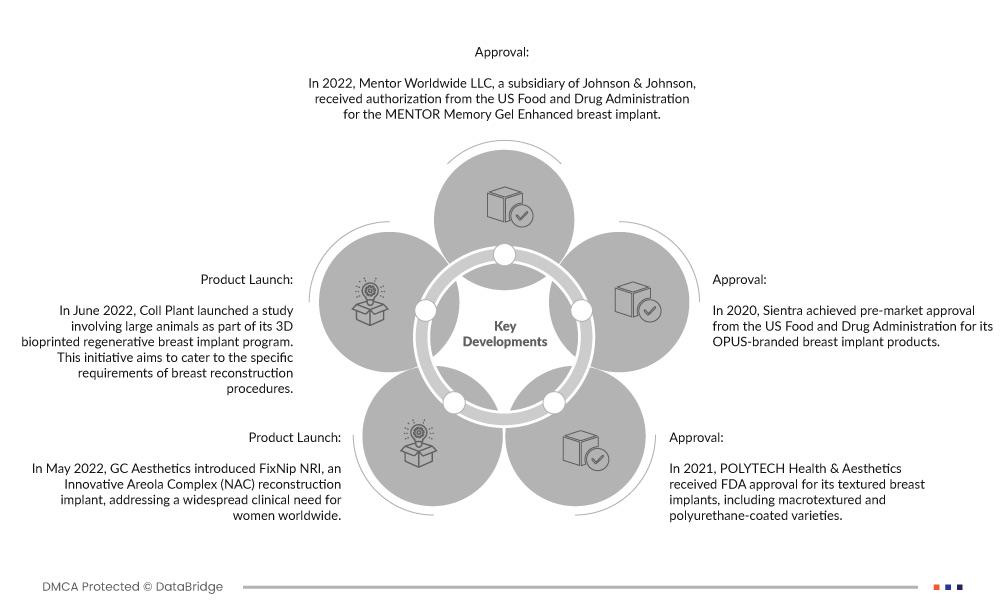
- В 2022 году Mentor Worldwide LLC, дочерняя компания Johnson & Johnson, получила разрешение от Управления по контролю за продуктами и лекарствами США на имплантат груди MENTOR Memory Gel Enhanced. Это одобрение знаменует собой новое дополнение к продуктовому портфелю Mentor, предоставляя пациентам, проходящим процедуру увеличения груди, инновационный и одобренный FDA вариант. Имплант Memory Gel Enhanced отражает приверженность компании предоставлению передовых решений по реконструкции и улучшению груди.
- В июне 2022 года Coll Plant начала исследование с участием крупных животных в рамках своей программы по 3D-биопечатным регенеративным грудным имплантатам. Эта инициатива направлена на удовлетворение особых требований процедур по реконструкции груди. Используя передовые технологии 3D-биопечати, Coll Plant стремится разрабатывать инновационные и регенеративные решения для грудных имплантатов, которые потенциально могут преобразовать область реконструкции груди, предлагая более естественные и персонализированные варианты для пациентов.
- В мае 2022 года компания GC Aesthetics представила FixNip NRI, инновационный имплантат для реконструкции ареолярного комплекса (NAC), отвечающий широко распространенной клинической потребности женщин во всем мире. Это медицинское устройство предлагает решение для выживших после рака груди и пациентов, желающих реконструировать сосково-ареолярный комплекс после мастэктомии. Предоставляя специализированный и инновационный имплантат, компания GC Aesthetics стремится улучшить качество жизни и эстетические результаты для значительного числа женщин.
- В 2021 году компания POLYTECH Health & Aesthetics получила одобрение FDA на свои текстурированные грудные имплантаты, включая макротекстурированные и покрытые полиуретаном разновидности. Это регулирующее одобрение стало важной вехой, позволившей компании предложить эти имплантаты на рынке США. Текстурированные имплантаты, известные своим естественным ощущением и эстетическими результатами, теперь доступны более широкому кругу пациентов, ищущих варианты увеличения и реконструкции груди, что расширяет возможности выбора и улучшает результаты в этой области.
- В 2020 году компания Sientra получила предварительное одобрение Управления по контролю за продуктами и лекарствами США на свои грудные имплантаты под брендом OPUS. Этот нормативный этап ознаменовал приверженность компании поставке высококачественных и безопасных вариантов грудных имплантатов на рынок США. С одобрением FDA имплантаты OPUS от Sientra стали надежным выбором для увеличения и реконструкции груди, предлагая пациентам и хирургам надежное и инновационное решение для эстетических и восстановительных процедур.
Более подробную информацию об отчете о рынке реконструкции груди в Европе можно получить здесь – https://www.databridgemarketresearch.com/reports/europe-breast-reconstruction-market