Market Definition and Insights
Syndromic multiplex diagnostic is a type of advanced diagnostic test utilised to detect infectious diseases such as respiratory infection, infective gastroenteritis, sexually transmitted infections, sepsis, and meningitis, among other types of infectious diseases. The syndromic multiplex diagnostic also helps the clinicians or hospitals to detect the symptoms and signs of the various types of diseases. This lets the health care providers provide the right treatment for the patients and offer more precise outcomes and care that can be performed more quickly.
Syndromic multiplex testing is used to diagnose many pathogens simultaneously. In the syndromic multiplex diagnostic, various types of reagents & consumables and instruments & accessories are used, which helps maintain accuracy and provide fast diagnosis results. These multiplex tests are rapidly diagnosed with certain infections, allowing clinical management decisions to be made promptly. The tests based on multiplex technology are known as test panels. The panels used in syndromic testing are designed to diagnose multiple diseases associated with the same or similar syndrome type. These panels help evaluate the cause of the disease at the point of care. Gastrointestinal panels and respiratory panels are the types of syndromic panels.
Syndromic multiplex testing utilizes the advanced technology of multiplex PCR which provides accurate and fast diagnostic results with the help of the multiple panels used in syndromic multiplex diagnostic to provide diagnostic results within an hour. The new generations of syndromic multiplex can rapidly identify the common type of pathogens in the respiratory specimens, blood and cerebrospinal. The use of multiplex panels is associated with quicker turnaround time, reduction of other unnecessary laboratory tests, faster diagnosis and targeted treatment.
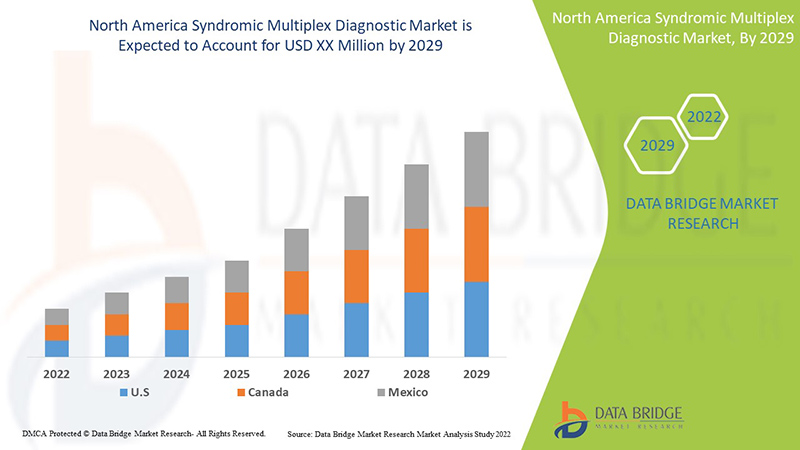
Data Bridge Market Research analyses that the North America syndromic multiplex diagnostic market will grow at a CAGR of 9.0% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product and Services (Reagents & Consumables, Instruments, Software & Accessories and Services), Infection Type (Viral, Bacterial, Parasites and Fungal), Disease (Respiratory Infections, Gastroenteritis, Sexually Transmitted Infections, Sepsis, Meningitis and Others), Panels Type (Respiratory Panel, GI-Enteric Panel, Sexually Transmitted Disease Panel, Blood-Sepsis Panel, Meningitis panel and Others), End User (Clinical Laboratories, Hospitals, Pharmaceutical & Biotechnology Companies, Research Institutes and Others) |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
BioFire Diagnostics (A Subsidiary of bioMérieux SA), Seegene Inc. (South Korea), Luminex Corporation. A DiaSorin Company (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), BD (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Cepheid (A subsidiary of Danaher (U.S.)), QIAGEN (Germany), Abbott (U.S.), Hologic, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Siemens Healthcare GmbH (Germany), Akonni Biosystems, Inc. (U.S.), Biocartis (Belgium), QuantuMDx Group Ltd. (U.K.), Applied BioCode, Inc. (U.S.), Prominex Inc. (U.S.), Nanomix, Inc. (U.S.), Curetis (A subsidiary of OpGen, Inc.) (Germany) |
Syndromic Multiplex Diagnostic Market Dynamics
Drivers
- Rise in prevalence of infectious diseases
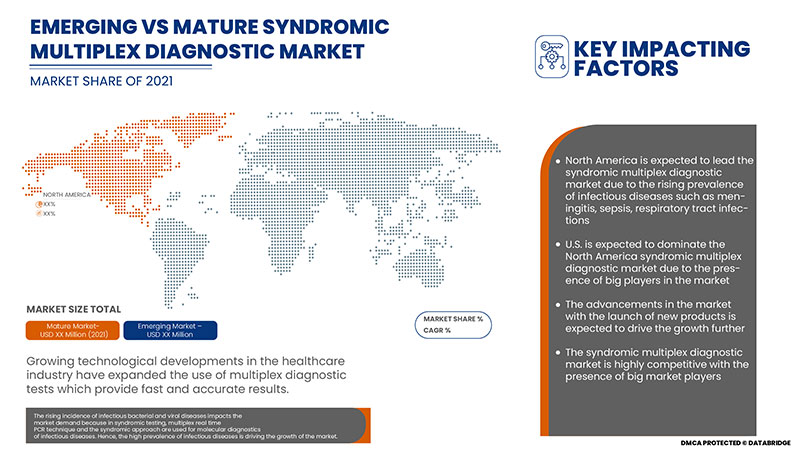
The rising incidence of infectious bacterial and viral diseases impacts the market's demand because, in the syndromic testing, multiplex real-time PCR technique and the syndromic approach are used for molecular diagnostics of infectious diseases.
- Increase in regulatory approval for acute respiratory syndrome coronavirus testing
In May 2020, biomérieux SA received FDA Emergency Use Authorization (EUA) for the BIOFIRE RP2.1 panel, which is used for the detection of 22 pathogens which is causing respiratory infections, including SARS-CoV-2 for COVID-19 disease.
The regulatory authorities, such as the FDA or CE mark, provide Emergency Use Authorization (EUA) approval for commercializing SARS-CoV-2 panels and testing to detect viruses associated with COVID-19 disease, which is a driver for the growth of the market.
Opportunities
- Strategic Initiatives taken by market players
In March 2021, F. Hoffman-La Roche Ltd acquired GenMark Diagnostics, a leading multiplex molecular diagnostics provider. This acquisition has helped the company to broaden Roche’s molecular diagnostic portfolio. These strategic initiatives taken by the market players, including focused segment product launches, are helping them expand their global reach and enhance their product portfolio and acting as an opportunity for the market's growth.
- Introduction of technologically advanced products
The syndromic multiplex testing utilizes the advanced technology of multiplex PCR to detect, isolate, or amplify targeted nucleic acid to provide accurate and fast diagnostic results; the advanced technology provides a diagnostic result within 45 to 60 min. Hence, the development of technologically advanced products is acting as an opportunity for the market's growth.
Restraint/Challenge
- High Cost of Diagnostic Products
The multiplex syndromic testing applications utilize the real-time Polymerase chain reaction (PCR), which delivers the results with amplification curves and correct values. The instruments used in the syndromic multiplex diagnostic require high maintenance costs. Hence, the high cost of instruments is a challenge for the market.
Post COVID-19 Impact on Syndromic Multiplex Diagnostic market
COVID-19 has positively affected the market. Market players are launching different products for the detection of the SARS-CoV virus. There is an increase in approval of products by regulatory bodies after post COVID-19, which leads to an increase in market growth.
Recent Development
- In March 2021, Luminex Corporation. A Diasorin Company received FDA Emergency Use Authorization and CE mark for expanded NxTAG respiratory panel test. This approval has helped the company to increase its revenue.
Syndromic Multiplex Diagnostic Market Scope
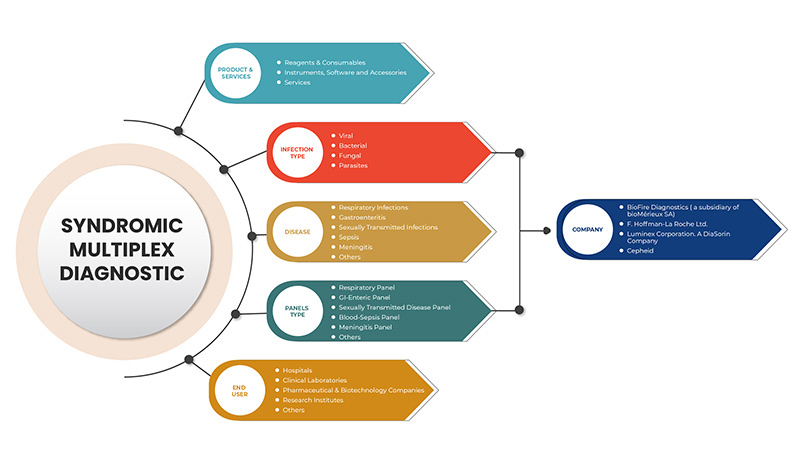
The syndromic multiplex diagnostic market is categorized into five segments: product and services, infection type, disease, panels type, and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product and Services
- Reagents & Consumables
- Instruments, Software & Accessories
- Services
Based on products and services, the North America syndromic multiplex diagnostic market is segmented into reagents & consumables, instruments, software & accessories and services.
Infection Type
- Viral
- Bacterial
- Parasites
- Fungal
Based on infection type, the North America syndromic multiplex diagnostic market is segmented into viral, bacterial, parasites and fungal.
Disease
- Respiratory Infection
- Gastroneteritis
- Sexually Transmitted Infections
- Sepsis
- Meningitis
- Others
Based on disease, the North America syndromic multiplex diagnostic market is segmented into respiratory infections, gastroenteritis, sexually transmitted infections, sepsis meningitis and others.
Panels Type
- Respiratory Panel
- GI-Enteric Panel
- Sexually Transmitted Disease Panel
- Blood-Sepsis Panel
- Meningitis Panel
- Others
Based on panels type, the North America syndromic multiplex diagnostic market is segmented into respiratory panel, GI-enteric panel, sexually transmitted disease panel, blood-sepsis panel, meningitis panel and others.
End User
- Hospitals
- Clinical Laboratories
- Pharmaceutical & Biotechnology Companies
- Research Institutes
- Others
Based on end user, the North America syndromic multiplex diagnostic market is segmented into clinical laboratories, hospitals, pharmaceutical & biotechnology companies, research institutes and others.
Syndromic Multiplex Diagnostic Market Regional Analysis/Insights
The syndromic multiplex diagnostic market is analysed and market size insights and trends are provided by country, product and services, infection type, disease, panels type and end user as referenced above.
The countries covered in the report are U.S., Canada, and Mexico.
The U.S. syndromic multiplex diagnostic market is expected to grow due to an increase in the prevalence of infectious diseases and a rise in demand for early and accurate diagnosis.
The country section of the report also provides individual market impacting factors and changes in regulations in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Syndromic Multiplex Diagnostic Market Share Analysis
The syndromic multiplex diagnostic market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Saudi Arabia presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to syndromic multiplex diagnostic market.
Some of the major players operating in the North America syndromic multiplex diagnostic market are BioFire Diagnostics (A Subsidiary of bioMérieux SA), Seegene Inc., Luminex Corporation. A DiaSorin Company, F. Hoffmann-La Roche Ltd, BD, Bio-Rad Laboratories, Inc., Cepheid (A subsidiary of Danaher), QIAGEN, Abbott, Hologic, Inc., Thermo Fisher Scientific Inc., Siemens Healthcare GmbH, Akonni Biosystems, Inc., Biocartis, QuantuMDx Group Ltd., Applied BioCode, Inc., Prominex Inc., Nanomix, Inc., Curetis (A subsidiary of OpGen, Inc.) among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT AND SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
4.3 MARKET SHARE PER PANEL, FOR TOP 3 PLAYERS (2021)
5 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF INFECTIOUS DISEASES
6.1.2 RISING ADOPTION OF MOLECULAR DIAGNOSTICS TECHNIQUES
6.1.3 INCREASING REGULATORY APPROVAL FOR SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-COV-2) TESTING
6.1.4 INCREASING DEMAND FOR FAST AND ACCURATE DIAGNOSTIC RESULTS
6.2 RESTRAINTS
6.2.1 HIGH COST OF DIAGNOSTIC PRODUCTS
6.2.2 EXPLICIT LIMITATION OF SYNDROMIC MULTIPLEX DIAGNOSTIC
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES TAKEN BY MARKET PLAYERS
6.3.2 RISING DIAGNOSTIC HEALTHCARE EXPENDITURE
6.3.3 INTRODUCTION OF TECHNOLOGICAL ADVANCED PRODUCTS
6.4 CHALLENGES
6.4.1 PRODUCT RECALLS
6.4.2 LACK OF SKILLED PROFESSIONALS AND BARRIERS FACED IN CONDUCTING DIAGNOSTIC TESTS
7 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT & SERVICES
7.1 OVERVIEW
7.2 REAGENTS & CONSUMABLES
7.3 INSTRUMENTS, SOFTWARE & ACCESSORIES
7.4 SERVICES
8 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE
8.1 OVERVIEW
8.2 VIRAL
8.2.1 CORONAVIRUS
8.2.2 INFLUENZA VIRUS
8.2.3 ADENOVIRUS
8.2.4 RHINOVIRUS
8.2.5 ROTAVIRUS
8.2.6 OTHERS
8.3 BACTERIAL
8.3.1 PNEUMONIAE
8.3.2 BORDETELLA PERTUSSIS
8.3.3 STAPHYLOCOCCUS
8.3.4 OTHERS
8.4 PARASITES
8.5 FUNGAL
9 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE
9.1 OVERVIEW
9.2 RESPIRATORY INFECTIONS
9.3 GASTROENTERITIS
9.4 SEXUALLY TRANSMITTED INFECTIONS
9.5 SEPSIS
9.6 MENINGITIS
9.7 OTHERS
10 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE
10.1 OVERVIEW
10.2 RESPIRATORY PANEL
10.3 GI-ENTERIC PANEL
10.4 SEXUALLY TRANSMITTED DISEASE PANEL
10.5 BLOOD-SEPSIS PANEL
10.6 MENINGITIS PANEL
10.7 OTHERS
11 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICAL LABORATORIES
11.4 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
11.5 RESEARCH INSTITUTES
11.6 OTHERS
12 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 BIOFIRE DIAGNOSTICS (A SUBSIDIARY OF BIOMÉRIEUX SA)
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 F. HOFFMANN-LA ROCHE LTD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 LUMINEX CORPORATION. A DIASORIN COMPANY
15.3.1 COMPANY SNAPSHOT
15.3.2 RECENT FINANCIALS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 CEPHEID (A SUBSIDIARY OF DANAHER)
15.4.1 COMPANY SNAPSHOT
15.4.2 RECENT FINANCIALS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 QIAGEN
15.5.1 COMPANY SNAPSHOT
15.5.2 RECENT FINANCIALS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 AKONNI BIOSYSTEMS, INC.
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 APPLIED BIOCODE, INC.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 BD
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 BIOCARTIS
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 BIO-RAD LABORATORIES, INC.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT
15.12 BOSCH HEALTHCARE SOLUTIONS GMBH (A SUBSIDIARY OF ROBERT BOSCH GMBH)
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 CURETIS (A SUBSIDIARY OF OPGEN, INC.)
15.13.1 COMPANY SNAPSHOT
15.13.2 RECENT FINANCIALS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 HOLOGIC, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 RECENT FINANCIALS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 MIRXES PTE LTD.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 NANŌMIX, INC.
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 PROMINEX INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 QUANTUMDX GROUP LTD.
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENTS
15.19 SEEGENE INC.
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 SIEMENS HEALTHCARE GMBH
15.20.1 COMPANY SNAPSHOT
15.20.2 RECENT FINANCIALS
15.20.3 PRODUCT PORTFOLIO
15.20.4 RECENT DEVELOPMENT
15.21 THERMOFISHER SCIENTIFIC INC.
15.21.1 COMPANY SNAPSHOT
15.21.2 RECENT FINANCIALS
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
List of Table
TABLE 1 COST OF THE PRODUCT
TABLE 2 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT & SERVICES, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA REAGENTS & CONSUMABLES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA INSTRUMENTS, SOFTWARE & ACCESSORIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA SERVICES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA PARASITES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA FUNGAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA RESPIRATORY INFECTIONS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA GASTROENTERITIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA SEXUALLY TRANSMITTED INFECTIONS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA SEPSIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA MENINGITIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA RESPIRATORY PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA GI-ENTERIC PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA SEXUALLY TRANSMITTED DISEASE PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA BLOOD-SEPSIS PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA MENINGITIS PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA HOSPITALS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA CLINICAL LABORATORIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA RESEARCH INSTITUTES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 41 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 42 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 43 U.S. VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 44 U.S. BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 45 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 46 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 47 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 49 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 50 CANADA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 51 CANADA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 52 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 53 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 54 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 56 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 57 MEXICO VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 58 MEXICO BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 59 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 60 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 61 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE AND INCIDENCE OF INFECTIOUS DISEASES IS EXPECTED TO DRIVE THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET IN THE FORECAST PERIOD
FIGURE 12 REAGENTS & CONSUMABLES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET
FIGURE 15 TOTAL CASES OF COVID-19 IN NORTH AMERICA
FIGURE 16 TOTAL CASES OF COVID-19 IN EUROPE
FIGURE 17 NATIONAL HEALTH EXPENDITURE VS MEDICAL DEVICE EXPENDITURE, U.S., 2019
FIGURE 18 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, 2021
FIGURE 19 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, 2022-2029 (USD MILLION)
FIGURE 20 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, CAGR (2022-2029)
FIGURE 21 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, LIFELINE CURVE
FIGURE 22 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, 2021
FIGURE 23 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, 2022-2029 (USD MILLION)
FIGURE 24 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, CAGR (2022-2029)
FIGURE 25 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, 2021
FIGURE 27 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, 2022-2029 (USD MILLION)
FIGURE 28 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, CAGR (2022-2029)
FIGURE 29 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, 2021
FIGURE 31 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, 2022-2029 (USD MILLION)
FIGURE 32 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, CAGR (2022-2029)
FIGURE 33 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, LIFELINE CURVE
FIGURE 34 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, 2021
FIGURE 35 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 36 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, CAGR (2022-2029)
FIGURE 37 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SNAPSHOT (2021)
FIGURE 39 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2021)
FIGURE 40 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2029)
FIGURE 41 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2021 & 2029)
FIGURE 42 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT AND SERVICES (2022-2029)
FIGURE 43 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.