North America Kidney Cancer Diagnostics Market Analysis and Size
Kidney cancer begins when healthy cells in one or both kidneys change and grow uncontrollably, forming a mass called a cortical tumor. The tumor can be malignant, indolent or benign. Malignancy is cancer, which means it can grow and spread to other parts of the body. An indolent tumor is also cancer, but this type of tumor rarely spreads to other parts of the body. A benign tumor means that the tumor can grow but not spread.
The rising awareness about kidney cancer North America has enhanced the demand for the market. The rising healthcare expenditure for better health services also contributes to the market's growth. The major market players focus on various service launches and approvals during this crucial period. In addition, the increase in improved diagnostic procedures for kidney cancer also contributes to the rising demand for kidney cancer diagnostics testing.
The North America kidney cancer diagnostics market is expected to grow in the forecast year due to the rise in market players and the availability of advanced services. Along with this, manufacturers are engaged in R&D activity for launching novel services in the market. The increasing research in the field of kidney diagnosis and development is expected to further boost the market growth. However, tissue damage due to high radiation exposure from imaging tests is expected to hamper the growth of the North America kidney cancer diagnostics market in the forecast period.
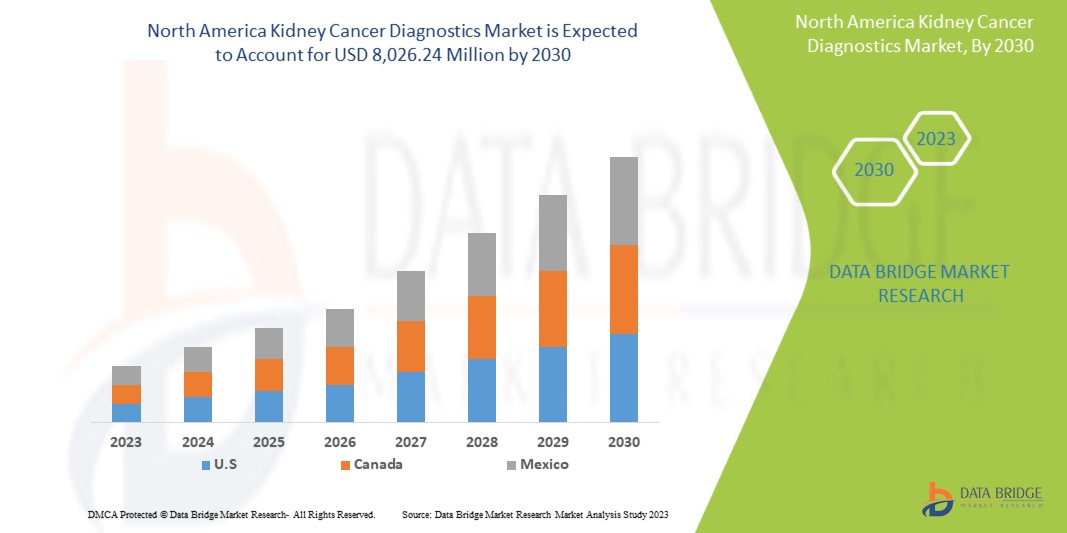
Data Bridge Market Research analyzes that the kidney cancer diagnostics market is expected to reach a value of USD 8,026.24 million by 2030, at a CAGR of 6.7% during the forecast period. Imaging accounts for the largest test type segment in the market due to the rising demand for smart devices, and increasing health expenditure has accelerated the demand for smart medical devices.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2020-2016) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
By Test Type (Imaging, Biomarker Test, Blood Test, Biopsy, Genetic Test, and Others), Cancer Stage (Stage I, Stage II, Stage III, and Stage IV), Tumor Type (Renal Cell Carcinoma, Clear Cell Renal Cell Carcinoma, Non Clear Cell Renal Cell Carcinoma), Product (Platform Based Products, Instrument Based Products, Kits and Reagents, and Other Consumables), Technology (Fluorescent In Situ Hybridization, Next Generation Sequencing, Fluorimmunoassay, Comparative Genomic Hybridization, Immunohistochemical, and Others), Application (Screening, Diagnostic and Predictive, Prognostic, and Research), End User (Hospitals, Diagnostic Centers, Cancer Research Centers, Academic Institutes, Ambulatory Surgical Centers, and Others), Distribution Channel (Direct Tender, Retail Sales, and Others). |
|
Countries Covered |
U.S., Canada, and Mexico |
|
Market Players Covered |
Siemens Healthcare GmbH, Koninklijke Philips N.V., FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, Thermo Fisher Scientific, Myriad Genetics, Inc., CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Illumina Inc., Ambry Genetics, Invitae Corporation, General Electric Company, Centogene N.V., GenPath, Creative Diagnostics, GeneDx LLC, Blueprint Genetics Oy, BioVendor R&D, and CD Genomics, and BD, among others |
Market Definition
Kidney cancer, commonly known as renal cancer, is a condition in which kidney cells develop into malignant (cancerous) tumors and expand uncontrollably. One of the top 10 most prevalent cancers is kidney cancer. Kidney cancer is fatal, and the diagnosis process also has safety issues; it is not cost-effective. Cancer patients may be hospitalized and receive a variety of therapies, such as surgery, radiation therapy, and systemic therapy. About 40% of kidney growths are tiny, localized masses. Localized refers to a tumor that has not spread from its original location. Kidney masses cannot be detected with regular laboratory procedures. Diagnosis of kidney cancer includes biopsy procedures, blood tests, and Imaging tests. Advanced kidney cancer therapies, like immunotherapy, radiation therapy, etc., are advised. Due to cutting-edge methods, nonsurgical procedures like cryoablation (which freezes cancer cells) and radiofrequency ablation are sometimes used to treat minor kidney tumors (heat cancer cells).
Kidney cancer can be difficult to diagnose because, despite its wide range of signs and symptoms, they are non-specific and can be linked to other, more widespread medical conditions. More than 43,000 men and 25,000 women are diagnosed with kidney and renal pelvis cancer each year, and 9,000 men and 5,000 women pass away as a result of this illness. However, strict regulations and standards for the approval and commercialization of kidney cancer diagnostic products are expected to restrain market growth.
North America Kidney Cancer Diagnostics Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- Growing Prevalence of Kidney Cancer
All ages can be affected by this type of cancer. Kidney cancer can be difficult to diagnose because, despite its wide range of signs and symptoms, they are non-specific and can be linked to other, more widespread medical conditions. Kidney cancer usually has no signs or symptoms in its early stages. Over time, signs and symptoms may occur, including blood in the urine, which may appear pink, red, or cola-colored, back or side pain that does not go away, loss of appetite, unexplained weight loss, fatigue, and fever. In adults, kidney cancer is the most common type of cancer. Young children are more likely to develop a type of kidney cancer called Wilms tumor. Renal cancer (also known as kidney cancer or renal cell adenocarcinoma) is the 14th most common cancer worldwide. It is 9th among men and 14th among women. In 2020, more than 30,000 new cases of kidney cancer were diagnosed.
Due to various risk factors such as smoking, obesity, high blood pressure (hypertension) or family history of kidney cancer, patients with kidney cancer is rising North America and becoming a significant socio-economic issue. Thus the rising number of patients with kidney cancer increases the demand for kidney cancer diagnostics products which act as a driver in the North America kidney cancer diagnostics market.
- Increase in Diagnostic Procedures for Kidney Cancer
Techniques used to diagnose kidney cancer include ultrasound, computerized tomography (CT) scans, magnetic resonance imaging (MRI) and, sometimes, positron emission tomography (PET) scans. Treatment for kidney cancer with a slow growth rate may involve monitoring. Chemotherapy for malignant is occasionally combined with radiation therapy and stem cell transplant. The increasing cancer rates have been a boosting factor for the increasing diagnostic product approval.
Thus, the rise in diagnostic product approvals has led to an increased number of highly efficient products in the market for kidney cancer diagnosis treatment. This is expected to act as a driver for the growth of the North America kidney cancer diagnostics market.
Opportunity
-
Rising Preference for Preventive Health Check-Ups
Preventive health check-up is preventive actions performed for the initial detection of kidney cancer disease. Also, a rising preference for preventive health check-ups provides a safeguard against likely exposure to any disease in the future.
Awareness to promote screening is the most important component of kidney cancer prevention. The check-up is comprised of the identification of cancer and examinations of risk factors to limit loss at an early stage.
Preventive kidney cancer check-up is performed with the help of various diagnostic test, which includes biopsy, immunohistochemistry, cancer screening, MRI and among others.
People are relatively more prone to kidney cancer diseases. Therefore, they require going through regular check-ups to facilitate physicians develop an understanding of diseases and to provide better treatment to a patient who is suffering from cancer, for the reason rising preference for preventive health check-ups is expected to act as a driver for the growth of North America kidney cancer diagnostics market.
Restraint/Challenge
- Strict Regulations And Standards For The Approval And Commercialization Of Kidney Cancer Diagnostic Products
The stringent regulations for the commercialization of any product in the market are proving to be a big challenge for the manufacturers of cancer diagnostic products North America that have regulations and a different body for the regulatory procedures.
Manufacturers first have to check the CE mark approval for their product's commercialization into the North America market. The stringent regulatory policies are expected to hinder the development of the cancer diagnostic market. The regulatory requirement for approvals of marketing or CE certification and application of laws and regulations could lead to major business changes or paying penalties, including the potential loss of business licenses. The resources and costs required to comply with these laws, rules, and regulations are high.
The regulatory requirement for marketing approvals, declaration of conformity, and the time required for regulatory review may vary for different products. The company which fails to get regulatory approval harms the business because without getting approval or failing to get CE to mark approval on the products, manufacturers are not able to launch their product in the North America market, and for this reason, strict regulations and standards for the approval and commercialization of kidney cancer diagnostic products are expected to act as a restrain for the North America kidney cancer diagnostics market.
Recent Developments
- In November 2022, Koninklijke Philips N.V. announced the North America launch of a next-generation compact portable ultrasound solution at the Radiological Society of North America (RSNA) annual meeting to bring the diagnostic quality associated with premium cart-based ultrasound systems to more patients. It is portable and versatile with good image quality or performance. It is Compatible with Philips ultrasound systems Affiniti and EPIQ transducer. This has helped the company to expand its product portfolio.
- In October 2022, General Electric Company had collaborated with several research institutes like the University of Cambridge Hospitals, Sophia Genetics, and earlier with Optellum to use imaging data in collaboration with Artificial intelligence. This will help to reduce the diagnosing time of several cancers and help to provide personalized care to patients. This has helped the company to widen its horizons in cancer diagnostics.
- In July 2022, Canon Medical Systems USA Inc. announced the completion of the acquisition of NXC Imaging, a medical imaging equipment distributor and service provider located in Minnesota, U.S. This results in expanding the service outreach in the North America market.
North America Kidney Cancer Diagnostics Market Scope
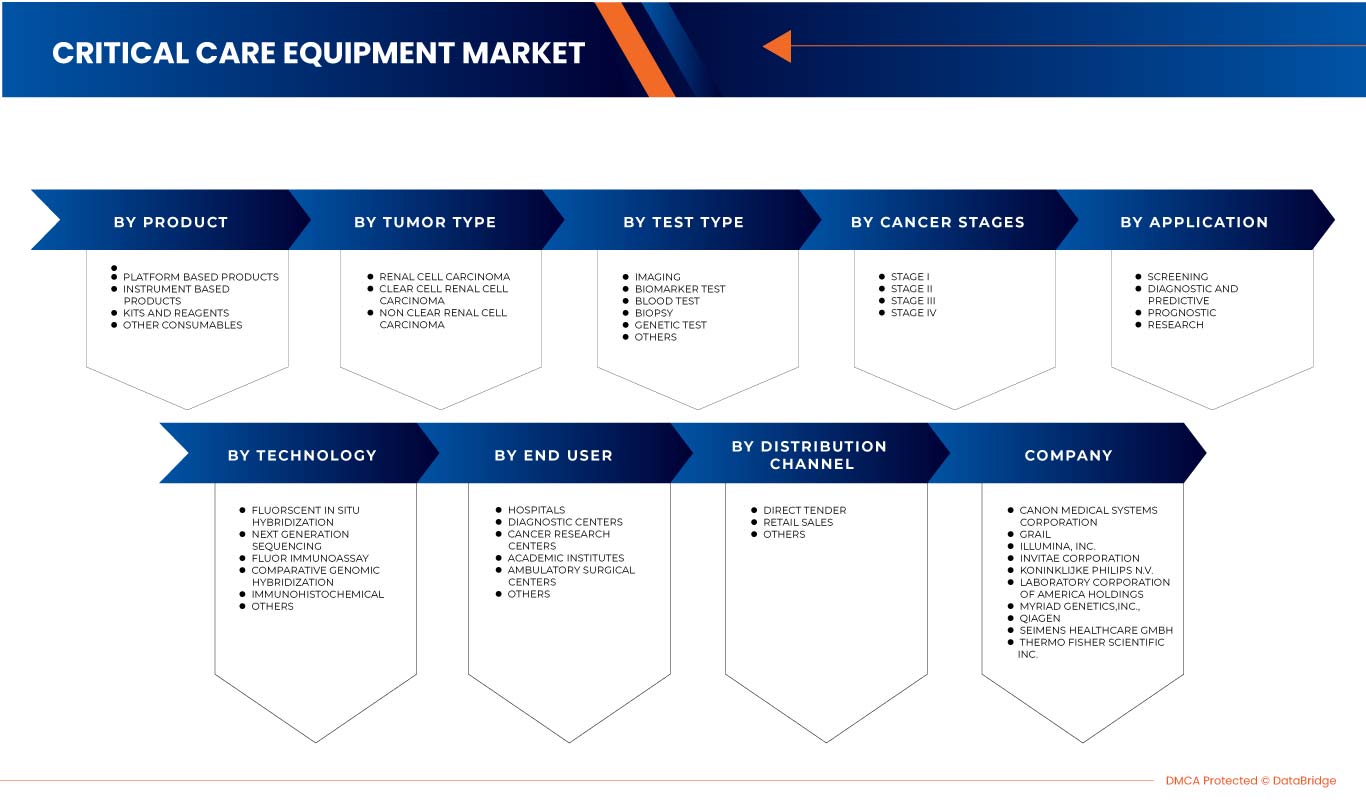
The North America kidney cancer diagnostics market is segmented into eight notable segments based on test type, cancer stage, tumor type, product, application, technology, end user and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
Test Type
- IMAGING TEST
- BIOMARKER TEST
- BLOOD TEST
- BIOPSY
- GENETIC TEST
- OTHERS
On the basis of test type, the North America kidney cancer diagnostics market is segmented into imaging, biomarker test, blood test, biopsy, genetic tests, and others.
Cancer Stage
- STAGE I
- STAGE II
- STAGE III
- STAGE IV
On the basis of cancer stage, the North America kidney cancer diagnostics market is segmented into stage I, stage II, stage III and stage IV.
Tumor Type
- RENAL CELL CARCINOMA
- CLEAR CELL RENAL CELL CARCINOMA
- NON-CLEAR CELL RENAL CELL CARCINOMA
On the basis of tumor type, the North America kidney cancer diagnostics market is segmented into renal cell carcinoma, Clear cell renal cell carcinoma, and non-clear cell renal cell carcinoma.
Product
- PLATFORM BASED PRODUCTS
- INSTRUMENT BASED PRODUCTS
- KITS AND REAGENTS
- OTHER CONSUMABLES
On the basis of product, the North America kidney cancer diagnostics market is segmented into instrument-based products, platform-based products, kits and reagents, and other consumables.
Technology
- FLUORESCENT IN SITU HYBRIDIZATION
- NEXT GENERATION SEQUENCING
- FLUOROIMMUNOASSAY
- COMPARATIVE GENOMIC HYBRIDIZATION
- IMMUNOHISTOCHEMICAL
- OTHERS
On the basis of technology, the North America kidney cancer diagnostics market is segmented into fluorescent in situ hybridization, next-generation sequencing, fluorimmunoassay, comparative genomic hybridization, immunohistochemical, and others.
Application
- SCREENING
- DIAGNOSTIC AND PREDICTIVE
- PROGNOSTIC
- RESEARCH
On the basis of application, the North America kidney cancer diagnostics market is segmented into screening, diagnostic and predictive, prognostic, and research.
End User
- HOSPITALS
- CANCER RESEARCH CENTERS
- ACADEMIC INSTITUTES
- DIAGNOSTIC CENTERS
- AMBULATORY SURGICAL CENTERS
- OTHERS
On the basis of end users, the North America kidney cancer diagnostics market is segmented into hospitals, diagnostic centers, cancer research centers, academic institutes, ambulatory surgical centers, and others.
Distribution Channel
- DIRECT TENDERS
- RETAIL SALES
- OTHERS
On the basis of distribution channel, the North America kidney cancer diagnostics market is segmented into direct tender, retail sales and others.
North America Kidney Cancer Diagnostics Market Regional Analysis/Insights
The North America kidney cancer diagnostics market is analyzed and market size information is provided based on country, test type, cancer stage, tumor type, product, application, technology, end user and distribution channel.
The countries covered in this market report are the U.S., Canada, and Mexico.
North America is dominating due to the presence of key market players in the largest consumer market with high GDP. The U.S. is expected to grow due to the rise in technological advancement in the healthcare industry.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Kidney Cancer Diagnostics Market Share Analysis
The kidney cancer diagnostics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product approvals, product width and breadth, application dominance, and product type lifeline curve. The above data points provided are only related to the company’s focus on the kidney cancer diagnostics market.
Some of the major players operating in the market are Siemens Healthcare GmbH, Koninklijke Philips N.V., FUJIFILM Corporation, Grail, Laboratory Corporation of America Holdings, Thermo Fisher Scientific, Myriad Genetics, Inc., CANON MEDICAL SYSTEMS CORPORATION, QIAGEN, Illumina Inc., Ambry Genetics, Invitae Corporation, General Electric Company, Centogene N.V., GenPath, Creative Diagnostics, GeneDx LLC, Blueprint Genetics Oy, BioVendor R&D, and CD Genomics, and BD, among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 TEST TYPE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
4.3 EPIDEMIOLOGY
4.3.1 KIDNEY CANCER INCIDENCES, 2020, BY BOTH SEXES
4.3.2 KIDNEY CANCER MORTALITY, 2020, BY BOTH SEXES
5 INDUSTRY INSIGHTS
6 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING PREVALENCE OF KIDNEY CANCER
7.1.2 INCREASE IN DIAGNOSTIC PROCEDURES FOR KIDNEY CANCER
7.1.3 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS AND TREATMENT
7.1.4 RISING AWARENESS TOWARDS KIDNEY CANCER
7.2 RESTRAINTS
7.2.1 STRICT REGULATIONS AND STANDARDS FOR THE APPROVAL AND COMMERCIALIZATION OF KIDNEY CANCER DIAGNOSTIC PRODUCTS
7.2.2 TISSUE DAMAGE DUE TO HIGH RADIATION EXPOSURE FROM IMAGING TESTS
7.3 OPPORTUNITIES
7.3.1 RISING PREFERENCE FOR PREVENTIVE HEALTH CHECK-UPS
7.3.2 GOVERNMENT INITIATIVES TOWARD KIDNEY CANCER DIAGNOSTICS
7.3.3 GROWING DEMAND FOR BETTER QUALITY HEALTHCARE
7.3.4 INCREASED DEMAND FOR NON-INVASIVE TESTING METHODS
7.4 CHALLENGES
7.4.1 LACK OF SKILLED AND CERTIFIED PROFESSIONALS
7.4.2 HIGH COST OF DIAGNOSTICS PROCEDURE FOR KIDNEY CANCERS
8 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 IMAGING
8.2.1 COMPUTED TOMOGRAPHY
8.2.2 ULTRASOUND
8.2.3 MAGNETIC RESONANCE IMAGING (MRI)
8.2.4 ANGIOGRAPHY
8.2.5 X-RAY
8.2.6 OTHERS
8.3 BLOOD TEST
8.4 BIOPSY
8.4.1 FINE NEEDLE ASPIRATION
8.4.2 NEEDLE CORE BIOPSY
8.5 BIOMARKER TEST
8.5.1 AQUAPORIN 1 (AQP1)
8.5.2 PERILIPIN (PLIN2)
8.5.3 N-METHYLTRANSFERASE (NMNT)
8.5.4 L-PLASTIN (LCP-1)
8.5.5 NM23A
8.6 GENETIC TEST
8.7 OTHERS
9 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE
9.1 OVERVIEW
9.2 STAGE I
9.3 STAGE II
9.4 STAGE III
9.5 STAGE IV
10 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE
10.1 OVERVIEW
10.2 RENAL CELL CARCINOMA
10.2.1 IMAGING
10.2.2 BLOOD TEST
10.2.3 BIOPSY
10.2.4 BIOMARKER TEST
10.2.5 GENETIC TEST
10.2.6 OTHERS
10.3 CLEAR CELL RENAL CELL CARCINOMA
10.3.1 IMAGING
10.3.2 BLOOD TEST
10.3.3 BIOPSY
10.3.4 BIOMARKER TEST
10.3.5 GENETIC TEST
10.3.6 OTHERS
10.4 NON CLEAR CELL RENAL CELL CARCINOMA
10.4.1 PAPILLARY RENAL CELL CARCINOMA
10.4.1.1 IMAGING
10.4.1.2 BLOOD TEST
10.4.1.3 BIOPSY
10.4.1.4 BIOMARKER TEST
10.4.1.5 GENETIC TEST
10.4.1.6 OTHERS
10.4.2 CHROMOPHOBE RENAL CELL CARCINOMA
10.4.2.1 IMAGING
10.4.2.2 BLOOD TEST
10.4.2.3 BIOPSY
10.4.2.4 BIOMARKER TEST
10.4.2.5 GENETIC TEST
10.4.2.6 OTHERS
11 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT
11.1 OVERVIEW
11.2 INSTRUMENT BASED PRODUCTS
11.2.1 IMAGING
11.2.2 BIOPSY
11.3 PLATFORM BASED PRODUCTS
11.3.1 NEXT GENERATION SEQUENCING
11.3.2 MICROARRAYS
11.3.3 PCR
11.3.4 OTHERS
11.4 KITS AND REAGENTS
11.4.1 RENAL CANCER PANELS
11.4.2 RENAL CANCER ANTIBODIES
11.5 OTHER CONSUMABLES
12 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY
12.1 OVERVIEW
12.2 FLUORESCENT IN SITU HYBRIDIZATION
12.3 NEXT GENERATION SEQUENCING
12.4 FLUORIMMUNOASSAY
12.5 COMPARATIVE GENOMIC HYBRIDIZATION
12.6 IMMUNOHISTOCHEMICAL
12.7 OTHERS
13 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION
13.1 OVERVIEW
13.2 SCREENING
13.2.1 INSTRUMENT BASED PRODUCTS
13.2.2 PLATFORM BASED PRODUCTS
13.2.3 KITS AND REAGENTS
13.2.4 OTHER CONSUMABLES
13.3 DIAGNOSTIC AND PREDICTIVE
13.3.1 INSTRUMENT BASED PRODUCTS
13.3.2 PLATFORM BASED PRODUCTS
13.3.3 KITS AND REAGENTS
13.3.4 OTHER CONSUMABLES
13.4 PROGNOSTIC
13.4.1 INSTRUMENT BASED PRODUCTS
13.4.2 PLATFORM BASED PRODUCTS
13.4.3 KITS AND REAGENTS
13.4.4 OTHER CONSUMABLES
13.5 RESEARCH
13.5.1 INSTRUMENT BASED PRODUCTS
13.5.2 PLATFORM BASED PRODUCTS
13.5.3 KITS AND REAGENTS
13.5.4 OTHER CONSUMABLES
14 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITALS
14.3 DIAGNOSTIC CENTERS
14.4 CANCER RESEARCH CENTERS
14.5 ACADEMIC INSTITUTES
14.6 AMBULATORY SURGICAL CENTERS
14.7 OTHERS
15 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
15.4 OTHERS
16 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
17 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 CANON MEDICAL SYSTEMS CORPORATION
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY PROFILE
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 KONINKLIJKE PHILIPS N.V.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY PROFILE
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 GENERAL ELECTRIC COMPANY
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY PROFILE
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.4 SIEMENS HEALTHCARE GMBH
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY PROFILE
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENT
19.5 GRAIL
19.5.1 COMPANY SNAPSHOT
19.5.2 PRODUCT PORTFOLIO
19.5.3 RECENT DEVELOPMENTS
19.6 AMBRY GENETICS
19.6.1 COMPANY SNAPSHOT
19.6.2 PRODUCT PORTFOLIO
19.6.3 RECENT DEVELOPMENT
19.7 BIOVENDOR R&D
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 BLUEPRINT GENETICS OY.
19.8.1 COMPANY SNAPSHOT
19.8.2 PRODUCT PORTFOLIO
19.8.3 RECENT DEVELOPMENT
19.9 CD GENOMICS
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENT
19.1 CENTOGENE N.V.
19.10.1 COMPANY SNAPSHOT
19.10.2 REVENUE ANALYSIS
19.10.3 PRODUCT PORTFOLIO
19.10.4 RECENT DEVELOPMENT
19.11 CREATIVE DIAGNOSTICS
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 FUJIFILM CORPORATION
19.12.1 COMPANY SNAPSHOT
19.12.2 REVENUE ANALYSIS
19.12.3 PRODUCT PORTFOLIO
19.12.4 RECENT DEVELOPMENTS
19.13 GENEDX, LLC
19.13.1 COMPANY SNAPSHOT
19.13.2 REVENUE ANALYSIS
19.13.3 PRODUCT PORTFOLIO
19.13.4 RECENT DEVELOPMENT
19.14 GENPATH, A DIVISION OF BIOREFERENCE LABORATORIES, AN OPKO HEALTH INC. COMPANY
19.14.1 COMPANY SNAPSHOT
19.14.2 PRODUCT PORTFOLIO
19.14.3 RECENT DEVELOPMENT
19.15 ILLUMINA, INC.
19.15.1 COMPANY SNAPSHOT
19.15.2 REVENUE ANALYSIS
19.15.3 PRODUCT PORTFOLIO
19.15.4 RECENT DEVELOPMENT
19.16 INVITAE CORPORATION
19.16.1 COMPANY SNAPSHOT
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.17 LABORATORY CORPORATION OF AMERICA HOLDINGS
19.17.1 COMPANY SNAPSHOT
19.17.2 REVENUE ANALYSIS
19.17.3 PRODUCT PORTFOLIO
19.17.4 RECENT DEVELOPMENTS
19.18 MYRIAD GENETICS, INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
19.19 THERMO FISHER SCIENTIFIC INC.
19.19.1 COMPANY SNAPSHOT
19.19.2 REVENUE ANALYSIS
19.19.3 PRODUCT PORTFOLIO
19.19.4 RECENT DEVELOPMENT
19.2 QIAGEN
19.20.1 COMPANY SNAPSHOT
19.20.2 REVENUE ANALYSIS
19.20.3 PRODUCT PORTFOLIO
19.20.4 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
List of Table
TABLE 1 ESTIMATED NEW CANCER CASES AND DEATHS
TABLE 2 APPROVED DIAGNOSTICS OF KIDNEY CANCER
TABLE 3 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA BLOOD TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA GENETIC TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA STAGE I IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA STAGE II IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA STAGE III IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA STAGE IV IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 31 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 32 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 35 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 36 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA OTHER CONSUMABLES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 42 NORTH AMERICA FLUORESCENT IN SITU HYBRIDIZATION IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA NEXT GENERATION SEQUENCING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA FLUORIMMUNOASSAY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA COMPARATIVE GENOMIC HYBRIDIZATION IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA IMMUNOHISTOCHEMICAL IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 49 NORTH AMERICA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA HOSPITALS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA DIAGNOSTIC CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA CANCER RESEARCH CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA ACADEMIC INSTITUTES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 64 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 65 NORTH AMERICA DIRECT TENDER IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 66 NORTH AMERICA RETAIL SALES IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA OTHERS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 83 NORTH AMERICA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 84 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 86 NORTH AMERICA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 87 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.S. IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 U.S. BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 U.S. BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 102 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 103 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 104 U.S. RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 105 U.S. CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 106 U.S. NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 107 U.S. PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 108 U.S. CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 109 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 110 U.S. INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 111 U.S. INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 112 U.S. INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 113 U.S. PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 114 U.S. PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 115 U.S. PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 116 U.S. KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 117 U.S. KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 118 U.S. KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 119 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 120 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 121 U.S. SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 122 U.S. DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 123 U.S. PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 124 U.S. RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 125 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 126 U.S. KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 127 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 128 CANADA IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 129 CANADA BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 130 CANADA BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 131 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 132 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 133 CANADA RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 CANADA CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 CANADA NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 136 CANADA PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 137 CANADA CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 139 CANADA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 140 CANADA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 141 CANADA INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 142 CANADA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 143 CANADA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 144 CANADA PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 145 CANADA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 146 CANADA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 147 CANADA KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 148 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 149 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 150 CANADA SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 151 CANADA DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 152 CANADA PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 153 CANADA RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 154 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 155 CANADA KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 156 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 157 MEXICO IMAGING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 158 MEXICO BIOPSY IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 159 MEXICO BIOMARKER TEST IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 160 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY CANCER STAGE, 2021-2030 (USD MILLION)
TABLE 161 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 162 MEXICO RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 163 MEXICO CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 MEXICO NON CLEAR CELL RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TUMOR TYPE, 2021-2030 (USD MILLION)
TABLE 165 MEXICO PAPILLARY RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 166 MEXICO CHROMOPHOBE RENAL CELL CARCINOMA IN KIDNEY CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 168 MEXICO INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 169 MEXICO INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 170 MEXICO INSTRUMENT BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 171 MEXICO PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 172 MEXICO PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 173 MEXICO PLATFORM BASED PRODUCTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 174 MEXICO KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 175 MEXICO KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (UNITS)
TABLE 176 MEXICO KITS AND REAGENTS IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD)
TABLE 177 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2021-2030 (USD MILLION)
TABLE 178 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY APPLICATION, 2021-2030 (USD MILLION)
TABLE 179 MEXICO SCREENING IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 180 MEXICO DIAGNOSTIC AND PREDICTIVE IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 181 MEXICO PROGNOSTIC IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 182 MEXICO RESEARCH IN KIDNEY CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 183 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 184 MEXICO KIDNEY CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF KIDNEY CANCER AND INCREASING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE GROWTH OF THE NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 THE IMAGING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET
FIGURE 14 KIDNEY CANCER INCIDENCE, BOTH SEXES, BY REGION (2020)
FIGURE 15 KIDNEY CANCER MORTALITY, BOTH SEXES, BY REGION (2020)
FIGURE 16 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2022
FIGURE 17 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 18 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, CAGR (2023-2030)
FIGURE 19 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TEST TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE , 2022
FIGURE 21 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, 2023-2030 (USD MILLION)
FIGURE 22 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, CAGR (2023-2030)
FIGURE 23 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY CANCER STAGE, LIFELINE CURVE
FIGURE 24 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, 2022
FIGURE 25 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, 2023-2030 (USD MILLION)
FIGURE 26 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, CAGR (2023-2030)
FIGURE 27 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TUMOR TYPE, LIFELINE CURVE
FIGURE 28 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, 2022
FIGURE 29 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 30 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, CAGR (2023-2030)
FIGURE 31 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY PRODUCT, LIFELINE CURVE
FIGURE 32 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, 2022
FIGURE 33 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, 2023-2030 (USD MILLION)
FIGURE 34 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, CAGR (2023-2030)
FIGURE 35 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY TECHNOLOGY, LIFELINE CURVE
FIGURE 36 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, 2022
FIGURE 37 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 38 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, CAGR (2023-2030)
FIGURE 39 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY APPLICATION, LIFELINE CURVE
FIGURE 40 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, 2022
FIGURE 41 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, 2023-2030 (USD MILLION)
FIGURE 42 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, CAGR (2023-2030)
FIGURE 43 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY END USER, LIFELINE CURVE
FIGURE 44 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2022
FIGURE 45 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 46 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 47 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 49 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 50 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 51 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 52 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: TEST TYPE (2023-2030)
FIGURE 53 NORTH AMERICA KIDNEY CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.