North America Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market, By Product (On-Premise Solutions, Cloud-Based Solutions, Web Based Solutions), Approach (Clinician Reported Outcome Assessment (ClinRO), Patient Reported Outcome Assessment (PRO), Observer Reported Outcome Assessment (ObsRO), Performance Outcome Assessment (PerfO)), End User (Commercial Service Providers, Hospitals and Transplant Centers, Research Laboratories, Academic Institutions), Platform (Contract Research Organizations, Pharmaceutical and Biopharmaceutical Companies, Medical Device Manufacturers, Hospitals and Clinical Laboratories, Consulting Service Companies, Research and Academia, Others), Countries (U.S., Canada and Mexico) Industry Trends and Forecast to 2028
Market Analysis and Insights: North America Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market

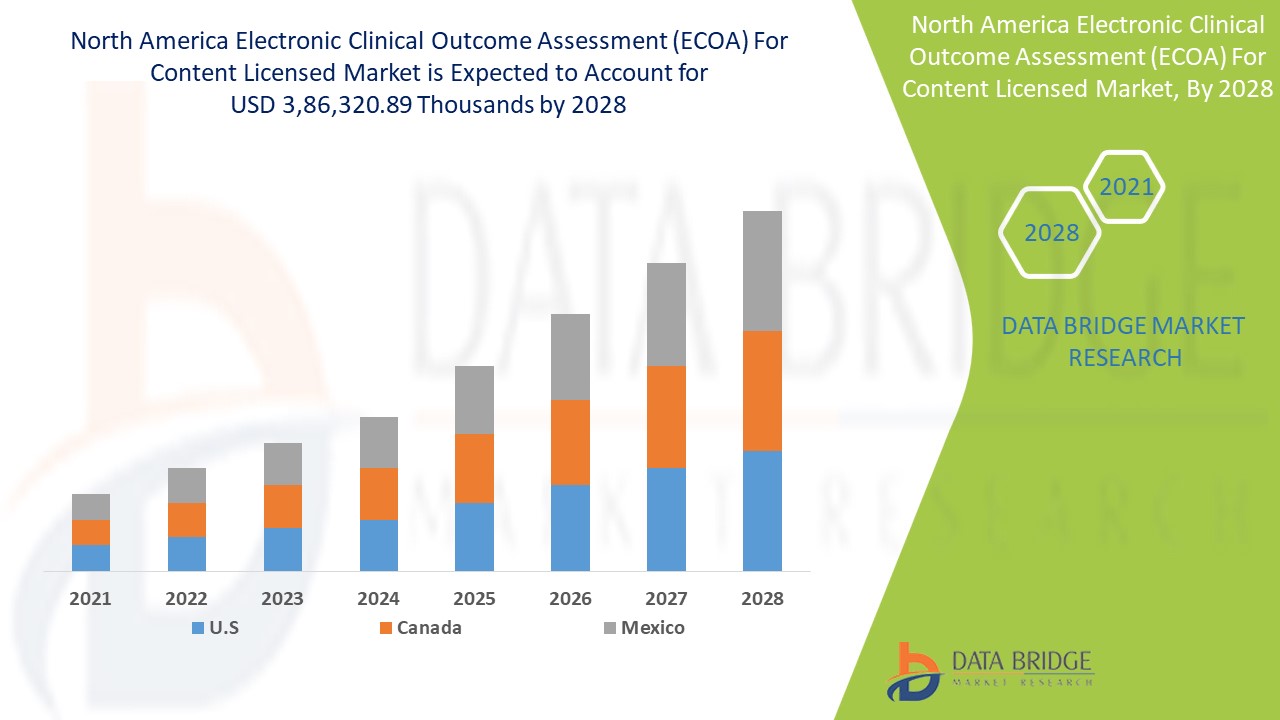
The electronic clinical outcome assessment (eCOA) for content licensed market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing with the CAGR of 15.1% in the forecast period of 2021 to 2028 and is expected to reach USD 3,86,320.89 thousands by 2028. The adoption of eCOA is being driven by a desire to engage more closely with patients by the pharma industry which is acting as a major driving factor for the electronic clinical outcome assessment (eCOA) for content licensed market.
Electronic clinical outcome assessment (eCOA) is the method of capturing data electronically in clinical trials. It can substantially increase the quality of study data while meeting regulatory requirements. eCOA employs technologies such as handheld devices, tablets or the web to allow trial participants, physicians and caregivers to directly report information related to healthcare outcomes.
Growing demand for eCOA due to its capability to collect large amount of data while simultaneously ensuring high quality is accelerating the electronic clinical outcome assessment (eCOA) for content licensed market. High initial financial costs associated with the implementation and utilization of these systems might restrain the electronic clinical outcome assessment (eCOA) for content licensed market. Rising levels of innovations and technological advancements (AI, ML, precision trials, precision-guided intervention, IoMT) is creating opportunities for the electronic clinical outcome assessment (eCOA) for content licensed market. Lack of well-trained professionals for accurate data interpretation is a major challenge for the electronic clinical outcome assessment (eCOA) for content licensed market.
This electronic clinical outcome assessment (eCOA) for content licensed market report provides details of market share, new developments, and product pipeline analysis, impact of domestic and localised market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographical expansions and technological innovations in the market. To understand the analysis and the electronic clinical outcome assessment (eCOA) for content licensed market scenario contact us for an Analyst Brief, our team will help you create a revenue impact solution to achieve your desired goal.
Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Scope and Market Size

The electronic clinical outcome assessment (eCOA) for content licensed market is segmented on the basis of product, approach, end user and platform. The growth among segments helps you analyse niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of product, the electronic clinical outcome assessment (eCOA) for content licensed market is segmented into on-premise solutions, cloud-based solutions and web based solutions. In 2021, on-premise segment holds the largest market share in electronic clinical outcome assessment (eCOA) for content licensed market as on-premise is the perpetual license with a fixed set of studies available for clients.
- On the basis of approach, the electronic clinical outcome assessment (eCOA) for content licensed market is segmented into clinician reported outcome assessment (ClinRO), patient reported outcome assessment (PRO), observer reported outcome assessment (ObsRO) and performance outcome assessment (PerfO). In 2021, clinician reported outcome assessment (ClinRO) segment holds the largest market share in electronic clinical outcome assessment (eCOA) for content licensed market as ClinRO measure involves a clinical judgment or interpretation of the observable signs, behaviours or other physical manifestations thought to be related to a disease or condition.
- On the basis of end user, the electronic clinical outcome assessment (eCOA) for content licensed market is segmented into commercial service providers, hospitals and transplant centers and research laboratories and academic institutions. In 2021, research laboratories and academic institutions segment holds the largest market share in electronic clinical outcome assessment (eCOA) for content licensed market as research organization and academic institution used broadly within the clinical and drug development industries, primarily refers to an academic and/or non-profit institution that performs one or more functions in the conduct of clinical trials.
- On the basis of platform, the electronic clinical outcome assessment (eCOA) for content licensed market is segmented into contract research organization, pharmaceutical and biopharmaceutical companies, medical device manufacturers, consulting service companies, hospitals and clinical laboratories, research and academia and others. In 2021, contract research organization segment holds the largest market share in electronic clinical outcome assessment (eCOA) for content licensed market as a contract research organization (CROs) is a service organization that provides support to the pharmaceutical and biotechnology industries in the form of outsourced pharmaceutical research services (for both drugs and medical devices).
North America Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Country Level Analysis
North America electronic clinical outcome assessment (eCOA) for content licensed market is analysed and market size information is provided by the country, product, approach, end user and platform.
The countries covered in the North America electronic clinical outcome assessment (eCOA) for content licensed market report are the U.S., Canada and Mexico.
The U.S. has been accounted for the largest market share as the region is witnessing high R&D spending of the pharmaceutical industry and increasing prevalence of diseases creating a demand for highly efficient pharmaceutical research and trials due to which the electronic clinical outcome assessment (eCOA) is dominating in the country. Canada is dominating with the second highest market share of due to the number of clinical trials conducted is very high as compared to other countries present in the region which is boosting the market in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Rising Number of Clinical Trials
The electronic clinical outcome assessment (eCOA) for content licensed market also provides you with detailed market analysis for every country growth in industry with sales, components sales, impact of technological development in electronic clinical outcome assessment (eCOA) for content licensed and changes in regulatory scenarios with their support for the electronic clinical outcome assessment (eCOA) for content licensed market. The data is available for historic period 2011 to 2019.
Competitive Landscape and Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Share Analysis
The electronic clinical outcome assessment (eCOA) for content licensed market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the companies’ focus related to North America electronic clinical outcome assessment (eCOA) for content licensed market.
The major players covered in the North America electronic clinical outcome assessment (eCOA) for content licensed market report are Oracle, IBM Corporation, Dassault Systemes, Parexel International Corporation, ERT Clinical, eClinical Solutions LLC, ArisGlobal, Clinical Ink, Kayentis, Anju Software, Inc., Signant Health, WIRB-Copernicus Group, YPrime LLC and Bioclinica among other domestic players. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Many product developments are also initiated by the companies worldwide which are also accelerating the growth of electronic clinical outcome assessment (eCOA) for content licensed market.
For instance,
- In February 2021, ERT Clinical, a leading global data and technology company for clinical endpoint data collection has introduced a powerful new solution eCOA Multimedia for enabling the collection, processing and analysis of photos and audio as part of clinical trial eCOA assessments. With this new launch, the company has increased its product line.
Partnership, joint ventures and other strategies enhances the company market share with increased coverage and presence. It also provides the benefit for organisation to improve their offering for electronic clinical outcome assessment (eCOA) for content licensed through expanded range of size.
SKU-

