Global Vascular Grafts And Peripheral Stents Market
Market Size in USD Billion
CAGR :
% 
 USD
3.94 Billion
USD
6.28 Billion
2024
2032
USD
3.94 Billion
USD
6.28 Billion
2024
2032
| 2025 –2032 | |
| USD 3.94 Billion | |
| USD 6.28 Billion | |
|
|
|
|
Vascular Grafts and Peripheral Stents Market Size
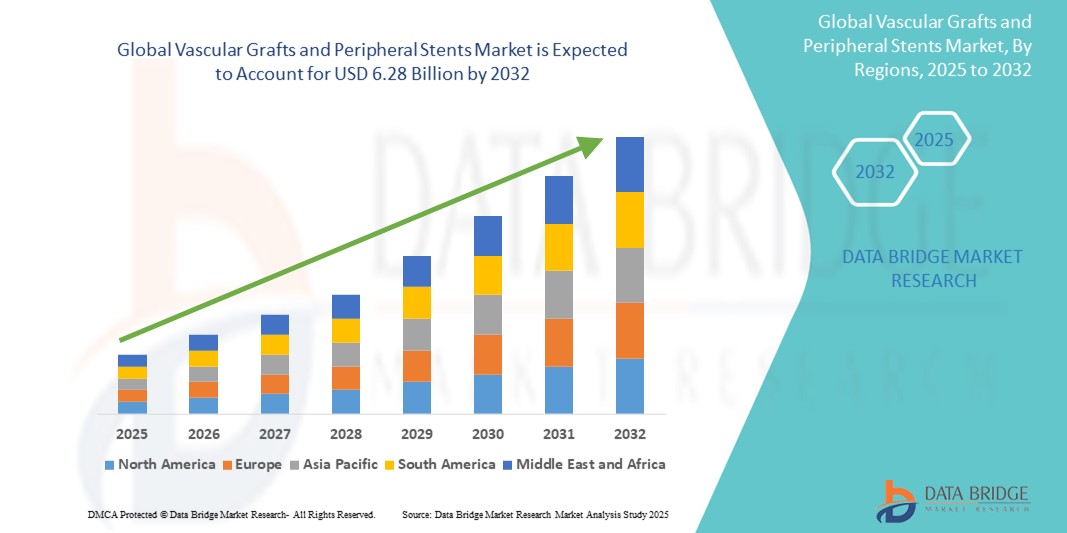
- The global vascular grafts and peripheral stents market size was valued at USD 3.94 billion in 2024 and is expected to reach USD 6.28 billion by 2032, at a CAGR of 6.00% during the forecast period
- The market growth is largely fuelled by the rising prevalence of cardiovascular diseases and increasing demand for minimally invasive procedures.
- Advances in biomaterials and technological innovations are also driving market expansion.
Vascular Grafts and Peripheral Stents Market Analysis
- The market for vascular grafts and peripheral stents is growing steadily, reflecting increased adoption of advanced medical devices in cardiovascular treatments. Innovations in design and materials are enhancing the safety and effectiveness of these products, making them preferred options in clinical settings
- Manufacturers are focusing on expanding product portfolios with improved durability and biocompatibility to meet evolving patient needs. The integration of cutting-edge technology is also streamlining procedures and supporting better patient outcomes
- North America dominates and holds the largest revenue share in the vascular grafts and peripheral stents market in 2024, driven by the high prevalence of cardiovascular diseases and a well-established healthcare infrastructure
- Asia-Pacific region is expected to witness the highest growth rate in the global vascular grafts and peripheral stents market, driven by rising healthcare expenditures, growing awareness of vascular diseases, and expanding elderly populations across countries such as China, India, and Japan
- The vascular graft segment holds a dominant market share in 2024 due to its extensive use in bypass surgeries and vascular reconstructions. These grafts are essential for restoring blood flow in blocked or damaged vessels, particularly in coronary and peripheral arteries
Report Scope and Vascular Grafts and Peripheral Stents Market Segmentation
|
Attributes |
Vascular Grafts and Peripheral Stents Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Vascular Grafts and Peripheral Stents Market Trends
“Increasing Adoption of Drug-Eluting and Bioengineered Vascular Grafts”
- There is a growing trend toward the use of drug-eluting vascular grafts and stents, which help reduce restenosis by releasing medication directly into the vessel walls.
- Bioengineered vascular grafts made from tissue-engineered materials are gaining traction due to their improved compatibility and reduced risk of rejection compared to synthetic options.
- These advanced grafts support faster healing and lower the need for repeat interventions, thus improving patient outcomes and reducing healthcare costs.
- For instance, companies such as Terumo and Gore Medical have developed drug-eluting stents that are widely adopted for peripheral artery disease treatments, enhancing efficacy.
- For instance, use of tissue-engineered grafts by Cytograft Tissue Engineering, which offer promising alternatives for bypass surgeries and show potential to transform vascular surgery standards.
- For instance, companies such as Terumo and Gore Medical have developed drug-eluting stents that are widely adopted for peripheral artery disease treatments, enhancing efficacy.
Vascular Grafts and Peripheral Stents Market Dynamics
Driver
“Rising Prevalence of Peripheral Artery Disease and Cardiovascular Disorders”
- The rising incidence of peripheral artery disease, caused by narrowed arteries that reduce blood flow to limbs, is significantly driving demand for vascular grafts and peripheral stents worldwide
- Factors such as aging populations, sedentary lifestyles, unhealthy diets, smoking, and obesity are contributing to the growing number of patients requiring vascular interventions
- Advancements in diagnostic technologies have improved early detection, allowing timely use of grafts and stents to manage arterial blockages and aneurysms more effectively
- The increasing prevalence of diabetes and hypertension, which worsen vascular health, further boosts the need for these medical devices
- Healthcare providers are investing in innovative, minimally invasive solutions to improve patient outcomes, exemplified by the adoption of drug-eluting stents that reduce restenosis rates in cardiovascular patients
Restraint/Challenge
“High Cost and Limited Reimbursement Policies”
- The high cost of vascular grafts and peripheral stents, driven by advanced technology, premium materials, and strict regulatory requirements, limits patient access globally
- Limited reimbursement coverage in many regions, especially in low- and middle-income countries, restricts affordability and adoption of these medical devices
- Expensive procedures and device costs create significant financial barriers, slowing market growth in emerging markets with constrained healthcare budgets
- Specialized surgical skills and hospital infrastructure needed for implantation add logistical challenges and increase overall treatment expenses
- These economic and systemic hurdles push manufacturers to focus on lowering costs and seeking improved reimbursement policies to expand market reach and patient access
Vascular Grafts and Peripheral Stents Market Scope
The market is segmented on the basis of product type, vascular graft raw material source, vascular graft indication, peripheral stent artery type, vascular graft application, and end users.
- By Product Type
On the basis of product type, the market is segmented into vascular graft and peripheral stent. The vascular graft segment holds a dominant market share in 2024 due to its extensive use in bypass surgeries and vascular reconstructions. These grafts are essential for restoring blood flow in blocked or damaged vessels, particularly in coronary and peripheral arteries.
The peripheral stent segment is expected to witness the fastest growth rate from 2025 to 2032, driven by increasing adoption in minimally invasive treatments. These stents are widely used to support blood vessels in various peripheral artery disease interventions. The technological improvements in stent flexibility and biocompatibility are further driving growth.
- By Vascular Graft Raw Material Source
On the basis of vascular graft raw material source, the market is segmented into synthetic, biosynthetic, and biological. The synthetic segment holds the largest market share in 2024, favored for its durability and wide availability in large-diameter vessel surgeries.
The biosynthetic grafts segment is expected to grow at the fastest rate from 2025 to 2032, driven by their hybrid composition that combines mechanical strength with better biological compatibility, supporting improved patient outcomes.
- By Vascular Graft Indication
On the basis of vascular graft indication, the market is segmented into endovascular aneurysm repair, peripheral vascular repair, and hemodialysis access. The endovascular aneurysm repair segment dominates the market share in 2024, driven by the rising incidence of abdominal aortic aneurysms and preference for less invasive procedures.
The peripheral vascular repair segment is expected to grow at the fastest rate from 2025 to 2032 due to the rising prevalence of peripheral artery disease and the increasing demand for minimally invasive vascular repair solutions.
- By Peripheral Stent Artery Type
On the basis of peripheral stent artery type, the market is segmented into carotid artery, fem-pop artery, iliac artery, and infrapop artery. The carotid artery segment accounts for the largest revenue share in 2024 due to its role in stroke prevention and expanding screening programs.
The fem-pop artery segment is expected to witness the fastest growth rate from 2025 to 2032 attributed to the high occurrence of lower limb arterial blockages requiring stenting procedures for revascularization.
- By Vascular Graft Application
On the basis of vascular graft application, the market is segmented into coronary artery disease, vascular occlusion, aneurysm, renal failure, and others. The coronary artery disease segment remains dominant in 2024, supported by the high global burden of cardiovascular disease and rising number of coronary artery bypass procedures.
The aneurysm and vascular occlusion segments is expected to witness the fastest growth rate from 2025 to 2032, as innovations in endovascular devices enhance the success and safety of treating complex vascular blockages and bulges.
- By End Users
On the basis of end users, the market is segmented into hospitals, ambulatory surgical centers, cardiac catheterization laboratories, specialty clinics, and others. The hospital segment commands the largest market share in 2024 owing to comprehensive treatment capabilities and a high volume of cardiovascular procedures.
The ambulatory surgical centers segment is expected to witness the fastest growth rate from 2025 to 2032, supported by the healthcare shift toward cost-effective outpatient care and the rising adoption of minimally invasive vascular interventions.
Vascular Grafts and Peripheral Stents Market Regional Analysis
- North America dominates and holds the largest revenue share in the vascular grafts and peripheral stents market in 2024, driven by the high prevalence of cardiovascular diseases and a well-established healthcare infrastructure
- The region's aging population and growing demand for minimally invasive vascular procedures are further fueling market growth. Increasing patient awareness, robust reimbursement frameworks, and technological innovations in endovascular treatments support adoption
- North America’s advanced clinical practices and early access to novel medical devices make it a key region for vascular interventions
U.S. Vascular Grafts and Peripheral Stents Market Insight
The U.S. accounted for the largest market revenue share within North America in 2024, supported by the high incidence of peripheral artery disease, diabetes, and chronic kidney disease. The country leads in vascular intervention volumes due to the availability of specialized centers and skilled vascular surgeons. Government and private investments in research and advanced graft and stent technologies further boost the market. In addition, rising health awareness and increased screening for vascular conditions contribute to strong market performance in the U.S.
Europe Vascular Grafts and Peripheral Stents Market Insight
The Europe is expected to witness the fastest growth rate from 2025 to 2032, attributed to the increasing burden of vascular disorders and the expansion of national healthcare programs. The region's focus on preventative care and widespread adoption of endovascular procedures in both public and private sectors support market growth. Strong clinical research infrastructure, supportive regulatory pathways, and a rise in lifestyle-related health issues are encouraging the use of vascular grafts and peripheral stents across Europe.
U.K. Vascular Grafts and Peripheral Stents Market Insight
The U.K. market is expected to witness the fastest growth rate from 2025 to 2032, driven by rising cases of arterial disease and growing investment in public health. The country's emphasis on early intervention and minimally invasive procedures contributes to higher demand for vascular products. Key market growth factors include enhanced diagnostic capabilities, increased funding for cardiovascular care, and initiatives aimed at improving access to advanced vascular interventions within the NHS and private healthcare systems.
Germany Vascular Grafts and Peripheral Stents Market Insight
The Germany is expected to witness the fastest growth rate from 2025 to 2032, owing to its aging population and high rate of cardiovascular complications. The country’s advanced hospital infrastructure and broad access to specialized vascular treatment centers play a pivotal role. Increased demand for biosynthetic and minimally invasive vascular repair options, supported by Germany's leadership in medical technology development, further drives adoption across both public and private healthcare settings.
Asia-Pacific Vascular Grafts and Peripheral Stents Market Insight
The Asia-Pacific is expected to witness the fastest growth rate from 2025 to 2032, driven by rising chronic disease prevalence, growing healthcare access, and expanding aging populations in countries such as China, Japan, and India. The region's healthcare infrastructure is rapidly improving, and increased investments in hospital capacity and medical technology are fueling demand. In addition, government-led health initiatives and the emergence of local manufacturers offering cost-effective graft and stent solutions are broadening access across APAC.
Japan Vascular Grafts and Peripheral Stents Market Insight
The Japan’s market is expected to witness the fastest growth rate from 2025 to 2032, driven by its advanced healthcare system and one of the world’s oldest populations, which has a high need for vascular interventions. Demand is supported by growing awareness of peripheral vascular disease and early adoption of cutting-edge medical technologies. Japan also emphasizes research and innovation, particularly in biologically compatible grafts and stents, which supports market advancement. Strong clinical outcomes and widespread access to vascular care enhance long-term growth prospects.
China Vascular Grafts and Peripheral Stents Market Insight
The China captured the largest market revenue share in Asia-Pacific in 2024, due to its rapidly aging population, rising lifestyle-related diseases, and healthcare system modernization. The country is investing heavily in cardiovascular treatment infrastructure and has seen a surge in local manufacturers producing vascular devices. Initiatives aimed at developing smart hospitals and expanding health insurance coverage are increasing procedural volumes. The growing middle class and demand for affordable, high-quality medical care continue to accelerate adoption.
Vascular Grafts and Peripheral Stents Market Share
The Vascular Grafts and Peripheral Stents industry is primarily led by well-established companies, including:
- GSK plc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Novartis AG (Switzerland)
- AstraZeneca (U.S.)
- Pfizer Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Sanofi (U.S.)
- Amgen Inc. (U.S.)
- DAIICHI SANKYO COMPANY, LIMITED. (Japan)
- AB Science (France)
- Eisai Co., Ltd. (Japan)
- Genentech, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
Latest Developments in Global Vascular Grafts and Peripheral Stents Market
-
In September 2023, Endovastec received registration approval from the Thailand Food and Drug Administration for three key products: the Castor Branched Aortic Stent-Graft and Delivery System, the Minos Abdominal Aortic Stent-Graft and Delivery System, and the Hercules Thoracic Stent Graft System with Low Profile Delivery System. These devices are designed for endovascular treatment of thoracic and abdominal aortic diseases, enhancing options for minimally invasive interventions and expanding the company’s presence in the Southeast Asian market.
- In August 2022, W. L. Gore & Associates, Inc. announced the acquisition of InnAVasc Medical, a medical technology company focused on improving care for patients with end-stage renal disease who depend on graft circuits for dialysis. This acquisition strengthens Gore’s portfolio in vascular access solutions, supporting innovation and better patient outcomes in the dialysis segment, while expanding its influence in the medical device market.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.