Global Transthyretin Amyloidosis Treatment Market, By Drug Type (Tafamidis, Patisiran, Inotersen, Others), Diseases Type (Hereditary Transthyretin Amyloidosis, aa), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) – Industry Trends and Forecast to 2030.
Transthyretin Amyloidosis Treatment Market Analysis and Size
Transthyretin amyloidosis results from transthyretin which is produced by the liver and forms dimers, that is followed by monomers. In familial amyloid polyneuropathy, the symptoms are first detected after the patient crosses 30 years of age, though it can also be detected in the early 20s or in the late 80s. Cardiac biomarkers such as N-terminal fragment of brain natriuretic peptide and troponin are present in abnormally high concentrations in the heart, lead to amyloid deposits that can be useful for further diagnosis of the disease. Symptoms may worsen if excess amyloid protein starts to collect in the nerves. Considering the fact timely symptom identification, diagnosis, and treatment are important for faster recovery of the patient.
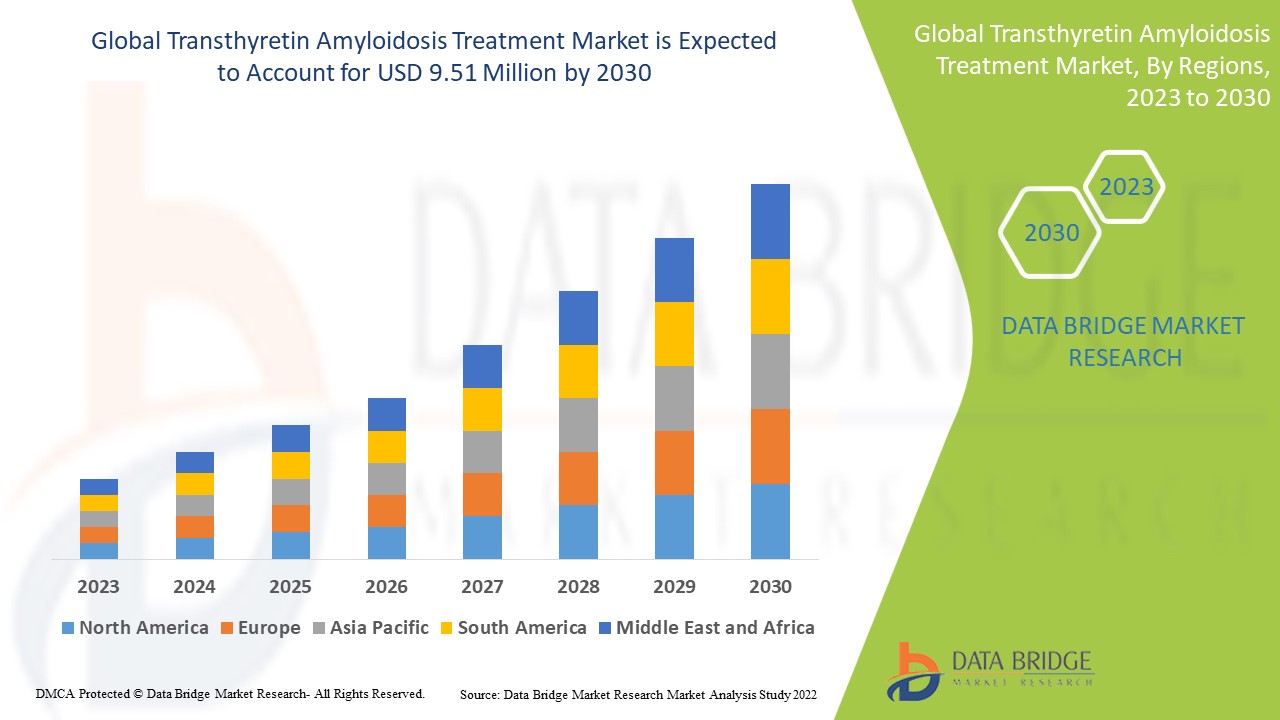
Data Bridge Market Research analyses a growth rate in the global transthyretin amyloidosis treatment market in the forecast period 2023-2030. The expected CAGR of the global transthyretin amyloidosis treatment market tends to be around 8.1% in the mentioned forecast period. The market was valued at USD 5.10 million in 2022 and would grow to USD 9.51 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Transthyretin Amyloidosis Treatment Market Scope and Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015 - 2020)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Drug Type (Tafamidis, Patisiran, Inotersen, Others), Diseases Type (Hereditary Transthyretin Amyloidosis, Wild Transthyretin Amyloidosis), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
|
|
Market Players Covered
|
Pfizer Inc (U.S.)., AstraZeneca (U.K.), Prothena Corporation plc (Ireland)., Ionis Pharmaceuticals (U.S.), BELLUS Health Inc (Canada)., Alnylam Pharmaceuticals, Inc (U.S.), Eidos Therapeutics, Inc (U.S.), SOM BIOTECH )Spain), Abbvie, Inc. (U.S.), Bausch Health Companies Inc. (U.S.)., Bristol Myers Squibb Company (U.S.) GSK Plc. (U.S.), Merck KGaA (Germany), Sanofi (France)
|
|
Market Opportunities
|
|
Market Definition
Transthyretin amyloidosis is a slowly progressive disease in which abnormal deposits of a protein called amyloid occur in the body's organs and tissues of the person. It is a progressive and fatal rare disease that can destroy nerve cells. It is often considered difficult to recognize and diagnose properly, and treatment is generally critical. However, the most common symptoms include heart failure, chronic diarrhea, weight loss, dry mouth, constipation, carpal tunnel syndrome, impaired kidney function, and floaters.
Global Transthyretin Amyloidosis Treatment Market Dynamics
Drivers
- Increase in the elderly population
The increasing elderly population in the country is expected to increase the incidence of transthyretin amyloidosis during the forecast period. According to the NCBI data, an age-related form of amyloidosis mainly affects the heart and is also known as wild-type ATTR. For instance, in 2020, about 727 million people worldwide were aged 65 years and above. It has been seen that the elderly population worldwide is growing significantly at a rate of 16.0% and is estimated to reach 1.5 billion in 2050. This factor enhances the market growth.
- Robust pipeline for transthyretin amyloidosis therapeutics
Several market players for the proper diagnosis and treatment of the disease are performing intensive R&D. For instance, in August 2022, Alnylam Pharmaceuticals, Inc. received FDA authorization for the drug Onpattro that could be used for nerve pain treatment caused by hereditary transthyretin amyloidosis. Furthermore, its phase III data shows that it has the potential to help patients suffering from cardiac-related issues for rare protein diseases.
Opportunities
- Increased initiatives by governmental bodies
Several initiatives undertaken by government and non-government bodies to create awareness associated with the disease and its treatment benefits among people are projected to boost early diagnosis of the disease, thus increasing the treatment rate. For instance, in May 2021, the Australian Amyloidosis Network organized a biennial touring workshop to create awareness about the disease among several healthcare professionals and patients with ATTR amyloidosis. The aim was to support the physicians and patients in understanding the disease's complexity, which will further help in developing new therapies for treating transthyretin amyloidosis.
- Increasing demand for targeted therapy
Targeted therapy is projected to witness the fastest growth during the forecast period due to increasing initiatives undertaken by major market players to support patients. For instance, Akcea Therapeutics, Inc. operates a patient assistance program called "Akcea Connect" to support hATTR amyloidosis patients. Under this program, the eligible person and their families get free, private, and personalized support across the U.S. This will increase awareness about available drug therapies, encouraging general practitioners to prescribe these drugs to transthyretin amyloidosis patients.
Restraints/Challenges
- Lack of skilled professionals
The lack of qualified healthcare professionals who cannot treat patients with appropriate treatment methods could limit the growth of the global transthyretin amyloidosis treatment market during a forecast period.
- High cost of transthyretin amyloidosis
High costs of recently launched drugs are projected to hinder the market growth. For instance, as per the reports of Alnylam Pharmaceutical, Inc., the cost of Patisiran is about US$ 450,000 annually before insurance. Another drug, Inotersen, which is manufactured by Ionis Pharmaceuticals and in partnership with Akcea Therapeutics, received approval for hereditary Transthyretin Amyloidosis Polyneuropathy indication, and the drug costs US$ 450,000 yearly.
This global transthyretin amyloidosis treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global transthyretin amyloidosis treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Transthyretin Amyloidosis Treatment Market
The COVID-19 pandemic negatively impacted the market growth due to the decrease in clinical trials for the drugs which could be used for the transthyretin amyloidosis treatment. For instance, in September 2020, a 74% reduction was witnessed in the average number of patients involved in clinical trials. Patients suffering from transthyretin amyloidosis have a higher risk of mortality and morbidity due to age and ATTR- amyloidosis-related organ dysfunction. The consequences of delayed diagnosis and interrupting treatment of patients should be balanced, considering the risk of exposure to the virus in the healthcare setting.
Recent Development
- In July 2019, Health Canada approved ONPATTRO for the treatment of hATTR with polyneuropathy in adults
Global Transthyretin Amyloidosis Treatment Market Scope
The global transthyretin amyloidosis treatment market is segmented on the basis of drug type, disease type, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Type
- Tafamidis
- Patisiran
- Inotersen
- Others
Diseases Type
- Hereditary Transthyretin Amyloidosis
- Wild Transthyretin Amyloidosis
End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Transthyretin Amyloidosis Treatment Market Regional Analysis/Insights
The global transthyretin amyloidosis treatment market is analyzed and market size insights and trends are provided by drug type, disease type, distribution channel and end-user as referenced above.
The major countries covered in the global transthyretin amyloidosis treatment market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Asia-Pacific has been witnessing positive growth for the global transthyretin amyloidosis treatment market throughout the forecasted period due to increased new research and developments on the transthyretin amyloidosis treatment market.
North America dominates the market due to the presence of key manufacturers and increasing research and development activities.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Transthyretin amyloidosis Treatment Market Share Analysis
The global transthyretin amyloidosis treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related global transthyretin amyloidosis treatment market
Key players operating in the global transthyretin amyloidosis treatment market include:
- Pfizer Inc (U.S.)
- AstraZeneca (U.K.)
- Prothena Corporation plc (Ireland)
- Ionis Pharmaceuticals (U.S.)
- BELLUS Health Inc (Canada)
- Alnylam Pharmaceuticals, Inc (U.S.)
- Eidos Therapeutics, Inc (U.S.)
- SOM BIOTECH (Spain)
- Abbvie, Inc. (U.S.)
- Bausch Health Companies Inc. (U.S.)
- Bristol Myers Squibb Company (U.S.)
- GSK Plc. (U.S.)
- Merck KGaA (Germany)
- Sanofi (France)
SKU-

