Global Respiratory Syncytial Virus (RSV) Diagnostic Market, By Product (Kits and Assays, Instruments, Others), Method (Molecular Diagnostics, Rapid Antigen Detection Test, Others), End User (Hospitals and Clinics, Clinical Laboratories, Others) – Industry Trends and Forecast to 2030.
Respiratory Syncytial Virus (RSV) Diagnostic Market Analysis and Size
The rise in the need for point-of-care testing diagnostic tests for RSV infected people, as well as advancements in the field of specific markers such as proteomics and genomics, are expected to drive the growth of the respiratory syncytial virus (RSV) diagnostic market during the forecast period. Furthermore, the rising prevalence of RSV infections is expected to fuel the expansion of the respiratory syncytial virus (RSV) diagnostic market.
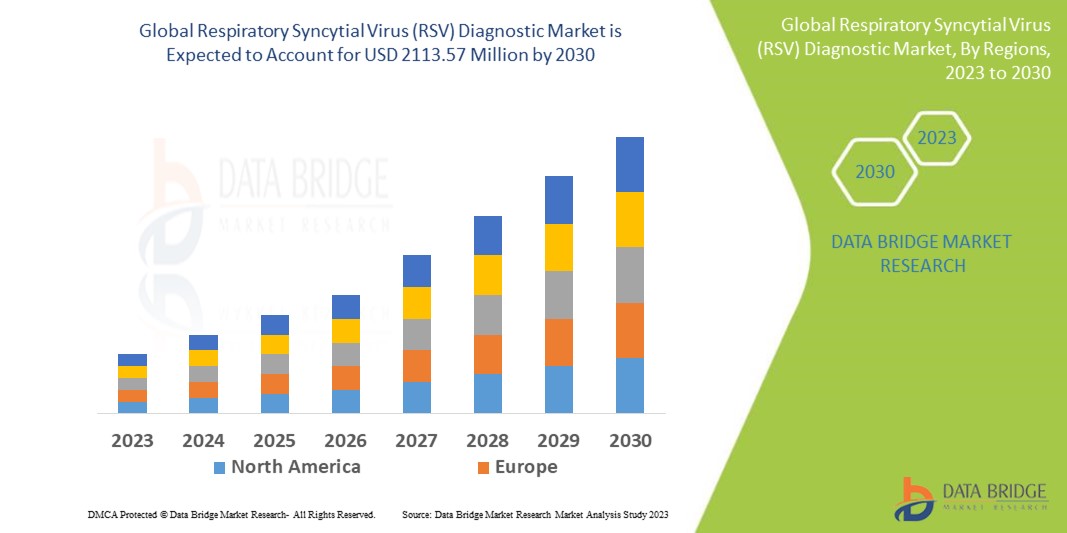
Data Bridge Market Research analyses that the respiratory syncytial virus (RSV) diagnostic market which is USD 923.10 million in 2022, is expected to reach USD 2113.57 million by 2030, at a CAGR of 10.91% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Respiratory Syncytial Virus (RSV) Diagnostic Market Scope and Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015 - 2020)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Product (Kits and Assays, Instruments, Others), Method (Molecular Diagnostics, Rapid Antigen Detection Test, Others), End User (Hospitals and Clinics, Clinical Laboratories, Others)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
|
|
Market Players Covered
|
BIOMERIEUX (France), BD (U.S.), Abbott (U.S.), F. Hoffman La. Roche Ltd (Switzerland), Danaher (U.S.), Thermo Fisher Scientific Inc. (U.S.), Luminex Corporation (U.S.), BioCartis, Hologic, Inc. (U.S.), Fast Track Diagnostics Luxembourg S.à r.l. (Luxembourg), Beckman Coulter Inc. (U.S.), Ortho-Clinical Diagnostics (U.S.), Bio-Rad Laboratories Inc. (U.S.) and DiaSorin S.p.A. (Italy)
|
|
Market Opportunities
|
|
Market Definition
RSV (respiratory syncytial virus) is a virus that causes respiratory infections like bronchiolitis and pneumonia. Human Respiratory Syncytial Virus (RSV) is a virus that commonly causes infection of the lower respiratory tract. RSV is a leading cause of severe respiratory illness in premature infants and children under five. Its symptoms can range from a simple cold to bronchiolitis and pneumonia.
Respiratory Syncytial Virus (RSV) Diagnostic Market Dynamics
Drivers
- Development of modern RSV detection systems
The development of modern RSV detection systems, such as western blot, enzyme-linked immunosorbent assay (ELISA), direct immunofluorescence, and real-time polymerase chain reaction, is being aided by advanced techniques such as bio- and nanotechnology (PCR). Besides this, governing agencies of numerous countries fund research and development (R&D) projects to develop new therapeutics. Other factors expected to drive the market include an increase in the number of clinical vaccine trials, an increase in the demand for in-vitro diagnostics, an increase in the incidence of childhood pneumonia, and an increase in the adoption of molecular diagnostics. These are the certain factors which propel the market growth.
- Rise in favourable reimbursement policies
RSV infection is more dangerous during the neonatal period, when children under the age of two develop severe respiratory symptoms. This, together with the growing emphasis of parents on child medical care, is one of the key factors driving market growth. Furthermore, the high survival rates after early diagnosis and the ease of access to diagnostic modalities act as growth-inducing factors. Aside from that, improving healthcare infrastructure and favourable reimbursement policies for hospitalised infants are also driving market growth.
- Rising demand of for early diagnosis
According to the 2021 article "Deaths Attributed to Respiratory Syncytial Virus in Young Children in High-Mortality Rate Settings: Report from Child Health and Mortality Prevention Surveillance (CHAMPS)," RSV was found in 5.5% of postmortem specimens. Furthermore, RSV was responsible for 6.5% of all infant deaths. This indicates that RSV is a significant burden on the global healthcare system, which increases demand for early diagnosis and drives market growth.
Opportunities
- New product launches
The product launches are propelling market growth. For instance, Avellino Labs USA, Inc. launched AvellinoCoV2 - Respiratory Test in 2022, a multi-panel RT-PCR-based virus assay that accurately detects four viral infections in a single patient sample - COVID-19, respiratory syncytial virus (RSV), influenza A and influenza B. Thus, the COVID-19 has had a positive impact on the growth of the market and is expected to continue to grow in the future.
Restraints/Challenges
- RSV vaccine advancements
RSV vaccine advancements may pose a further challenge to the growth of the respiratory syncytial virus (RSV) diagnostic market in the near future.
This respiratory syncytial virus (RSV) diagnostic market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the respiratory syncytial virus (RSV) diagnostic market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In 2021, QIAGEN launched and CE-marked the QIAstat-Dx Respiratory 4 Plex Flu A-B/RSV/SARS-CoV-2 test for the QIAstat-Dx system to rapidly identify whether patients have common seasonal respiratory infections or SARS-CoV-2.
- In 2021, Roche launched three molecular polymerase chain reaction (PCR) diagnostic test panels to detect and differentiate common respiratory pathogens, including influenza A, influenza B, and respiratory syncytial virus (RSV); adenovirus (ADV), human metapneumovirus (hMPV), and enterovirus/rhinovirus (EV/RV); and parainfluenza 1, 2, 3, and 4.
Global Respiratory Syncytial Virus (RSV) Diagnostic Market Scope
The respiratory syncytial virus (RSV) diagnostic market is segmented on the basis of product, method and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Kits and Assays
- Instruments
- Others
Method
- Molecular Diagnostics
- Rapid Antigen Detection Test
- Enzyme-linked immunosorbent assay
- Immunefluorescence assays
- Others
- Chromatographic immunoassays
- Optical immunoassays
End User
- Hospitals and Clinics
- Clinical Laboratories
- Others
Respiratory Syncytial Virus (RSV) Diagnostic Market Regional Analysis/Insights
The respiratory syncytial virus (RSV) diagnostic market is analyzed and market size insights and trends are provided by country, product, method and end user as referenced above.
The countries covered in the respiratory syncytial virus (RSV) diagnostic market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the respiratory syncytial virus (RSV) diagnostic market because these infections are increasing. Furthermore, the region's growing infant population and increasing number of strategic partnerships between pharmaceutical majors and in vitro diagnostic manufacturing companies will propel the respiratory syncytial virus (RSV) diagnostic market during the forecast period.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 due to the rise in the population density and the growing incidence of infectious diseases. Furthermore, the region's advancing healthcare systems and rising number of government initiatives are expected to drive the growth of the respiratory syncytial virus (RSV) diagnostic market in the coming years.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The respiratory syncytial virus (RSV) diagnostic market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for respiratory syncytial virus (RSV) diagnostic market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the respiratory syncytial virus (RSV) diagnostic market. The data is available for historic period 2011-2021.
Competitive Landscape and Respiratory Syncytial Virus (RSV) Diagnostic Market Share Analysis
The respiratory syncytial virus (RSV) diagnostic market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to respiratory syncytial virus (RSV) diagnostic market.
Some of the major players operating in the respiratory syncytial virus (RSV) diagnostic market are:
- BIOMERIEUX (France)
- BD (U.S.)
- Abbott (U.S.)
- F. Hoffman La. Roche Ltd (Switzerland)
- Danaher (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Luminex Corporation (U.S.)
- BioCartis, Hologic, Inc. (U.S.)
- Fast Track Diagnostics Luxembourg S.à r.l. (Luxembourg)
- Beckman Coulter Inc. (U.S.)
- Ortho-Clinical Diagnostics (U.S.)
- Bio-Rad Laboratories Inc. (U.S.)
- DiaSorin S.p.A. (Italy)
SKU-