Global Post Acute Myocardial Infarction Market, By Drug Class (Antiplatelet Therapy, Beta Blockers, Renin-Angiotensin-Aldosterone System Inhibitors, Statin Therapy, and Others), End-Users (Hospitals, Homecare, Specialty Clinics, and Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy) – Industry Trends and Forecast to 2031.
Post Acute Myocardial Infarction Market Analysis and Size
According to the National Library of Medicine U.S., about 70% of fatal cases of AMI are linked to blockages caused by atherosclerotic plaques. Since atherosclerosis is the primary cause of AMI, efforts to prevent the disease often focus on reducing risk factors associated with it. Modifiable risk factors contribute to 90% of AMI cases in men and 94% in women. These factors, which can be changed or controlled, include smoking, lack of physical activity, high blood pressure, obesity, high levels of cholesterol (especially LDL), and elevated triglyceride levels. On the other hand, age, gender, and family history are nonmodifiable risk factors for atherosclerosis and AMI.
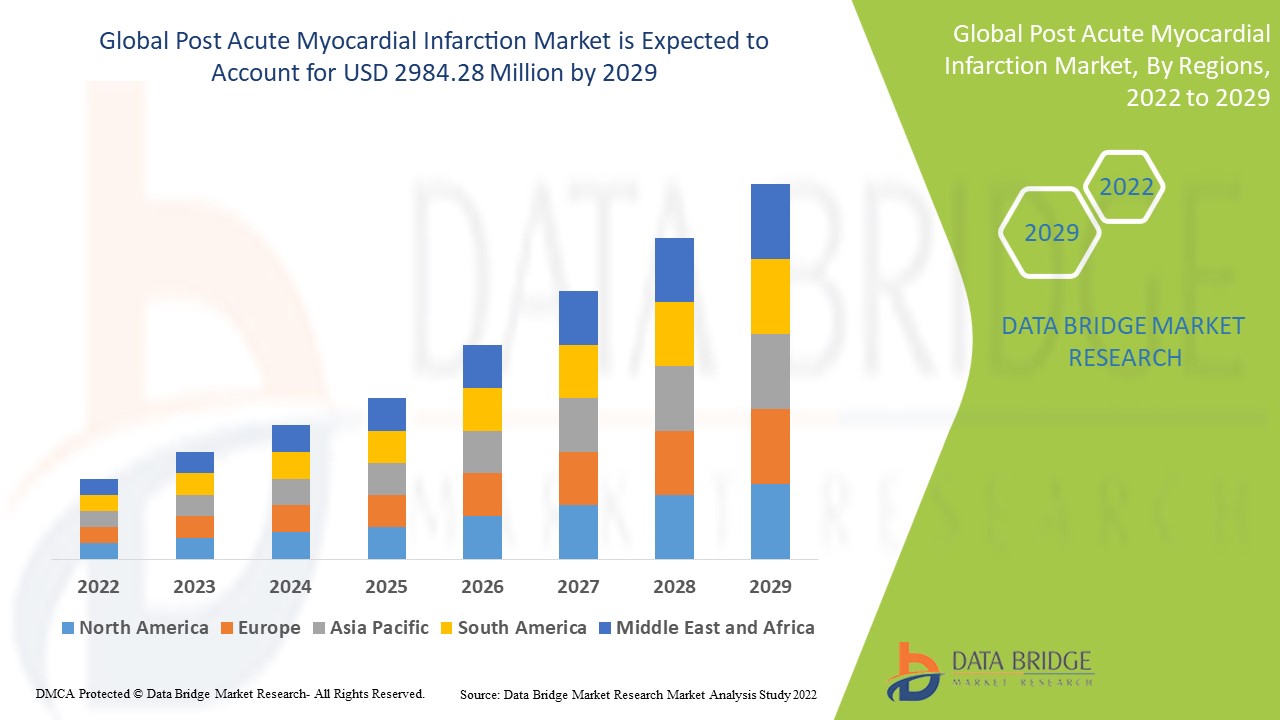
The global post acute myocardial infarction market size was valued at USD 2.05 billion in 2023 and is projected to reach USD 3.37 billion by 2031, with a CAGR of 6.4% during the forecast period of 2024 to 2031. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Years
|
2022 (Customizable to 2016-2021)
|
|
Quantitative Units
|
Revenue in USD Billion, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Drug Class (Antiplatelet Therapy, Beta Blockers, Renin-Angiotensin-Aldosterone System Inhibitors, Statin Therapy, and Others), End-Users (Hospitals, Homecare, Specialty Clinics, and Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, and Rest of South America
|
|
Market Players Covered
|
F. Hoffmann-La Roche Ltd (Switzerland), Fresenius Kabi AG (Germany), Bayer AG (Germany), Sun Pharmaceutical Industries Ltd (India), Novartis AG (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd (Israel), Zydus Group (India), Pfizer Inc (U.S.), Lupin (India), GSK Plc (U.K.), Glenmark Pharmaceuticals Inc (India), Amneal Pharmaceuticals (U.S.), Alnylam Pharmaceuticals, Inc (U.S.), and DAIICHI SANKYO COMPANY, LIMITED (Japan)
|
|
Market Opportunities
|
|
Market Definition
Post-acute myocardial infarction (AMI), commonly known as a heart attack, refers to the period following the acute phase of a heart attack. The post-acute phase is characterized by ongoing recovery, rehabilitation, and managing the patient's condition to prevent future cardiac events. This phase typically involves lifestyle modifications, such as dietary changes, exercise programs, and smoking cessation, as well as medication management to control risk factors such as hypertension, hyperlipidemia, and diabetes.
Post Acute Myocardial Infarction Market Dynamics
Drivers
- Increasing Prevalence of Cardiovascular Diseases
The prevalence of cardiovascular diseases, including acute myocardial infarction (AMI), is on the rise globally. Sedentary lifestyles, unhealthy dietary habits, and an aging population are contributing factors to the increasing incidence of these diseases. Sedentary lifestyles, characterized by low levels of physical activity, are linked to obesity, hypertension, and other risk factors for cardiovascular diseases. As the population ages, the risk of developing cardiovascular diseases increases, further driving the market growth.
- Advancements in Treatment Options
Technological advancements and innovations in treatment options for AMI have significantly improved patient outcomes and reduced the risk of complications. One such advancement is the development of drug-eluting stents, which help prevent restenosis (the re-narrowing of arteries) after angioplasty. Minimally invasive procedures, such as percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG), have also become more refined, leading to shorter recovery times and improved patient outcomes. Personalized medicine, which involves tailoring treatment plans based on individual patient characteristics, has emerged as a promising approach to treating AMI. These advancements in treatment options are driving market growth.
Opportunities
- Rising Healthcare Expenditure
With growing economies and aging populations, there is a greater focus on improving healthcare infrastructure and access to medical services. This is fueling the need for sophisticated treatments and technologies for conditions such as AMI. Moreover, the rise in disposable incomes and the expansion of the middle class in numerous developing nations are boosting healthcare expenditure. As a result, there is a growing market for AMI treatments in these regions, presenting opportunities for pharmaceutical companies, medical device manufacturers, and healthcare providers to expand their presence and offerings.
- Integration of Digital Health Solutions
Integrating digital health solutions, such as telemedicine, remote monitoring, and mobile health apps, is revolutionizing the management of AMI. These digital solutions enable healthcare providers to monitor patients with AMI more efficiently and cost-effectively, facilitating the delivery of timely and personalized care. Telemedicine, in particular, has emerged as a valuable tool for remote consultation and monitoring patients with AMI, reducing the need for in-person visits and improving patient outcomes. Furthermore, using mobile health apps for self-management and education has empowered patients to take a more active role in their healthcare. Overall, the integration of digital health solutions enhances patient care and management of AMI while creating new opportunities for innovation and growth in the market.
Restraints/Challenges
- Risk of Complications
Despite advancements in treatment options, treatments for acute myocardial infarction (AMI) carry a risk of complications that can deter patients from seeking treatment. These complications can include bleeding, infection, and cardiac arrhythmias, among others. Bleeding may occur as a side effect of anticoagulant medications used to prevent blood clotting, a common complication of AMI. Infection can occur due to the invasive nature of some treatments, such as angioplasty or coronary artery bypass grafting (CABG). Cardiac arrhythmias, or irregular heartbeats, can occur due to damage to the heart muscle during AMI. These complications can be serious and require additional medical intervention, which can be daunting for patients and may lead them to avoid or delay seeking treatment for AMI, which can hinder the market growth.
- Stringent Regulatory Requirements
Stringent regulatory requirements are implemented to guarantee the safety, effectiveness, and quality of new treatments and devices. Companies must conduct extensive clinical trials to demonstrate the safety and efficacy of their products, which can be time-consuming and costly. In addition, regulatory agencies may require additional data or studies before approving a new treatment or device, further delaying market entry. These regulations for approving new treatments and devices for AMI can pose a challenge for companies in the market.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In November 2022, Abiomed, a leading provider of cardiovascular technologies, agreed to be acquired by Johnson & Johnson for USD 16.6 billion. Upon completing the transaction, Abiomed will enhance Johnson & Johnson MedTech's position as a leading cardiovascular innovator and will continue to operate as a separate business entity. This acquisition aims to advance the standard of care for heart failure, a serious disease, and improve patient outcomes
- In August 2022, late-breaking research presented in a Hot Line session revealed that administering Asundexian 50 mg to post-myocardial infarction patients inhibited factor XIa by more than 90% without a significant increase in bleeding. Asundexian and other factor XIa inhibitors could be promising new therapies for reducing ischaemic events without significantly increasing bleeding in patients following myocardial infarction and in other clinical settings where vascular thrombosis or thromboembolism are concerns
Post Acute Myocardial Infarction Market Scope
The market is segmented on the basis of drug class, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- Antiplatelet Therapy
- Beta Blockers
End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Post Acute Myocardial Infarction Market Regional Analysis/Insights
The market is analyzed and market size insights and trends are provided by country, drug class, distribution channel, and end-user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, and Rest of South America.
North America is expected to dominate the market due to high prevalence of myocardial infarction, driving the demand for effective treatment options. In addition, North America boasts significant advancements in treatment technologies, including cutting-edge medical devices and pharmaceuticals, which further propel market growth. Moreover, the presence of well-established healthcare infrastructure and the adoption of advanced healthcare practices in the region contribute to its dominant position in the market.
Asia-Pacific is expected to witness significant growth during the forecast period due to the market being dominated by a vulnerable aging population that is particularly susceptible to cardiovascular disorders. This demographic trend has contributed significantly to the region's dominance in the market.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the market. The data is available for historic period 2011-2021.
Competitive Landscape and Post Acute Myocardial Infarction Market Share Analysis
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Some of the major players operating in the market are:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Fresenius Kabi AG (Germany)
- Bayer AG (Germany)
- Sun Pharmaceutical Industries Ltd (India)
- Novartis AG (Switzerland)
- Mylan N.V. (U.S.)
- Teva Pharmaceutical Industries Ltd (Israel)
- Zydus Group (India)
- Pfizer Inc (U.S.)
- Lupin (India)
- GSK Plc (U.K.)
- Glenmark Pharmaceuticals Inc (India)
- Amneal Pharmaceuticals (U.S.)
- Alnylam Pharmaceuticals, Inc (U.S.)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
SKU-