Global Nucleic Acid Based Drugs Market, By Category (Antisense, siRNA, miRNA, Aptamer, Decoy, CpG-oilgo), Structure (Single-Stranded DNA/RNA, Double-Stranded DNA, Single-Stranded DNA, Others), Target (Transcriptor Factor, TLR9-Receptor, Protein), Mechanism (Inhibits the Physiological Effect, Adjuvant, Inhibits Transcription, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2030.
Nucleic Acid Based Drugs Market Analysis and Size
Novel treatment techniques based on nucleic acid medicines are being developed. Due to unique properties that allow them to address undruggable targets using traditional small molecule or protein/antibody-based biologics, they promise to treat human diseases such as malignancies, viral infections, and genetic abnormalities. Nucleic acid medications use nucleotide sequence information to influence the biological processes of cells. These medications act either because of their expression in cells or because of the control of genes, particularly those with complementary sequences.
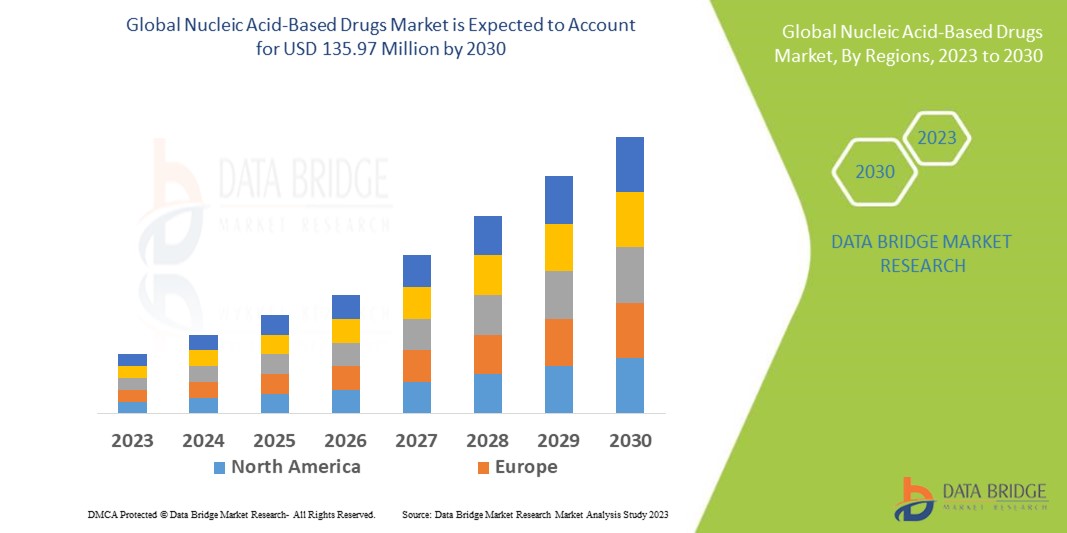
Data Bridge Market Research analyzes that the global nucleic acid-based drugs market, which was USD 32.05 million in 2022, is likely to reach USD 135.97 million by 2030 and is expected to undergo a CAGR of 19.80% during the forecast period. This indicates that the market value. “Antisense” dominates the category segment of the Nucleic Acid Based Drugs market owing to surging drug approvals and launches. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Nucleic Acid Based Drugs Market Scope and Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2016-2030)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Category (Antisense, siRNA, miRNA, Aptamer, Decoy, CpG-oilgo), Structure (Single Stranded DNA/RNA, Double Stranded DNA, Single-Stranded DNA, Others), Target (Transcriptor Factor, TLR9-Receptor, Protein), Mechanism (Inhibits the Physiological Effect, Adjuvant, Inhibits Transcription, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America
|
|
Market Players Covered
|
Wave Life Sciences Ltd. (U.S.), Copernicus Therapeutics Inc. (U.S.), Imugene (Australia), PYC Therapeutics (Australia), Protagonist Therapeutics Inc. (U.S.), Benitec Biopharma (Australia), Egen, Inc. (U.S.), Biomedica Medizinprodukte GmbH (UK), Transgene (France), Arrowhead Pharmaceuticals, Inc. (U.S.), Ionis Pharmaceuticals (U.S.), Alnylam Pharmaceuticals, Inc. (U.S.), Moderna, Inc. (U.S.), Gotham Therapeutics (U.S.), Sumitomo Chemical Co., Ltd. (Japan), Eli Lilly and Company (U.S.)
|
|
Market Opportunities
|
|
Market Definition
Target therapeutics based on nucleic acids or closely related chemical substances are known as nucleic acid-based drugs. These medications have the ability to target disorders at the genetic level and prevent disease-causing proteins from being expressed. The major active elements in nucleic acids are oligonucleotides, made via chemical synthesis, and tiny molecules. Nucleic acids are usually thought of as drug transporters, and they can cause undesired biological responses such immune system activation and blood clotting pathway extension. As a result, the advantages of nucleic acid-based pharmaceuticals in possible drug delivery systems for controlled drug releases are the primary rationale for their increased use in the market.
Nucleic Acid Based Drugs Market Dynamics
Drivers
- Rising incidence of genetic and rare diseases
The increasing prevalence of genetic and rare diseases, such as cystic fibrosis, muscular dystrophy, and various inherited genetic disorders, has created a significant demand for nucleic acid-based therapies. These drugs offer the potential to treat the underlying genetic causes of these diseases, providing hope for patients who previously had limited treatment options.
- Growing investment in research and development
Pharmaceutical companies and biotechnology firms are investing heavily in research and development of nucleic acid-based drugs. This includes antisense oligonucleotides, siRNA, and gene therapies. The promise of these therapies in addressing previously untreatable conditions has spurred increased funding and innovation in the field.
- Advancements in biotechnology and genomics
Ongoing advancements in biotechnology and genomics have led to a better understanding of nucleic acids, including DNA and RNA. This has paved the way for the development of novel nucleic acid-based drugs. The ability to sequence and manipulate nucleic acids has accelerated drug discovery and development in this field.
- Personalized medicine and targeted therapies
Nucleic acid-based drugs can be tailored to an individual's genetic makeup, enabling personalized treatment plans. This approach is gaining prominence as it can lead to more effective and safer treatments, particularly in cancer and rare diseases. Advances in precision medicine are driving the demand for nucleic acid-based therapies.
Opportunities
- Expansion of rare disease therapies
Nucleic acid-based drugs have shown immense promise in treating rare genetic disorders that were previously untreatable. As more research and development efforts focus on these conditions, there is an opportunity to provide life-changing treatments for patients with rare diseases. This market segment is relatively underserved, creating a significant growth opportunity.
- Advances in gene editing technologies
Advances in gene editing technologies like CRISPR-Cas9 are opening up new possibilities for gene therapies. This technology allows for precise modification of genes, offering opportunities to correct genetic mutations that underlie various diseases. The development and commercialization of gene editing-based nucleic acid therapies present a significant growth avenue.
Restraints
- High development costs
The research and development of nucleic acid-based drugs are often costly and time-consuming. The need for extensive preclinical and clinical trials, as well as the complexities of delivery systems and gene editing technologies, contribute to high development costs. This can be a significant barrier for smaller biotechnology companies and startups looking to enter the market.
- Safety concerns and regulatory hurdles
Nucleic acid-based therapies can have unique safety concerns, such as off-target effects and immune responses. These safety issues may pose challenges in gaining regulatory approvals and market acceptance. Regulatory agencies often have strict requirements for the safety and efficacy of novel therapies, which can delay market entry.
Challenges
- Delivery system complexity
Efficient and targeted delivery of nucleic acids to specific cells or tissues in the body is a complex challenge. Developing delivery systems that can protect the therapeutic nucleic acids, facilitate their entry into target cells, and avoid degradation is a major obstacle. Overcoming this challenge is crucial for the success of nucleic acid-based drugs.
- Immunogenicity and safety concerns
Nucleic acid-based drugs, especially gene therapies, can trigger immune responses or have off-target effects. Ensuring the safety and minimizing adverse reactions is a significant challenge. Researchers and developers must work to enhance the safety profiles of these therapies to gain regulatory approvals and patient trust.
This nucleic acid based drugs market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the nucleic acid based drugs market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- In March 2023, Ionis Pharmaceutical received a unanimous vote from the Food and Drug Administration (FDA) advisory committee for the potential accelerated approval of Tofersen for SOD1-ALS. Tofersen is an antisense oligonucleotide that mediates the degradation of superoxide dismutase 1 (SOD1) messenger RNA to reduce SOD1 protein synthesis
- In February 2023, Myeloid Therapeutics Inc. collaborated with New South Wales (NSW) Government in Australia to develop a state-of-the-art GMP manufacturing facility focused on RNA immunotherapies. The new facility would accelerate the commercialization of Myceloid's RNA therapeutics and the construction of an RNA ecosystem in the NSW
Global Nucleic Acid Based Drugs Market Scope
The Nucleic Acid Based Drugs market is segmented on the basis of category, structure, target, mechanism, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Category
- Antisense
- SiRNA
- MiRNA
- Aptamer
- Decoy
- CpG-oilgo
Structure
- Single Stranded DNA/RNA
- Double Stranded DNA
- Single-Stranded DNA
- Others
Target
- Transcriptor Factor
- TLR9-Receptor
- Protein
Mechanism
- Inhibits the Physiological Effect
- Adjuvant
- Inhibits Transcription
- Others
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Nucleic Acid Based Drugs Market Regional Analysis/Insights
The nucleic acid based drugs market is analysed and market size insights and trends are provided by country, category, structure, target, mechanism, end-users and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada, Mexico, Germany, U.K., Italy, France, Spain, Russia, Turkey, Switzerland, Belgium, Netherlands, Denmark, Sweden, Poland, Turkey, rest of Europe, Japan, China, South Korea, India, Australia, Singapore, Japan, Thailand, Indonesia, New Zealand, Vietnam, Thailand, Indonesia, Malaysia, Philippines, rest of Asia-Pacific, South Africa, Kuwait, Qatar, Oman, Saudi Arabia, U.A.E., and rest of the Middle East and Africa, Brazil, Argentina & rest of South America.
North America is expected to dominate the nucleic acid-based drugs market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the presence of major key players and rising healthcare expenditure will further propel the market's growth rate in this region. Additionally, increasing research and development activities will further propel the market's growth rate in this region.
Asia-Pacific is expected to grow during the forecast period of 2023-2030 due to the surging number of research and development activities in this region. Also, the development of healthcare infrastructure will further propel the market's growth rate in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The nucleic acid based drugs market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for Nucleic Acid Based Drugs market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the Nucleic Acid Based Drugs market. The data is available for historic period 2010-2020.
Competitive Landscape and Nucleic Acid Based Drugs Market Share Analysis
The nucleic acid-based drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to nucleic acid-based drugs market.
Some of the major players operating in the nucleic acid-based drugs market are:
- Eli Lilly and Company (U.S.)
- Wave Life Sciences Ltd. (U.S.)
- Copernicus Therapeutics Inc. (U.S.)
- Imugene (Australia)
- PYC Therapeutics (Australia)
- Protagonist Therapeutics Inc. (U.S.)
- Benitec Biopharma (Australia)
- Egen, Inc. (U.S.)
- Biomedica Medizinprodukte GmbH (U.K.)
- Transgene (France)
- Arrowhead Pharmaceuticals, Inc. (U.S.)
- Ionis Pharmaceuticals (U.S.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Moderna, Inc. (U.S.)
- Gotham Therapeutics (U.S.)
- Sumitomo Chemical Co., Ltd. (Japan)
SKU-