Global Medical Device Ethylene Oxide Sterilization Market
Market Size in USD Billion
CAGR :
% 
 USD
2.18 Billion
USD
3.23 Billion
2024
2032
USD
2.18 Billion
USD
3.23 Billion
2024
2032
| 2025 –2032 | |
| USD 2.18 Billion | |
| USD 3.23 Billion | |
|
|
|
|
Medical Dynamometer Market Analysis
According to the article published by AdvaMed in 2023, Ethylene oxide (EtO) sterilization is thepreferred method for approximately 50 percent of all medical device types, with over 20 billion devices sterilized annually. Its effectiveness in sterilizing a wide range of medical devices, including those with complex geometries and heat-sensitive materials, makes it a vital part of modern healthcare. Most surgeries involve at least one device that has been sterilized using Ethylene oxide (EtO), ensuring patient safety and infection control. In compliance with FDA's Quality System regulation and international standards, manufacturers must validate their Ethylene oxide (EtO) sterilization processes to guarantee they meet the highest levels of safety and efficacy, further underscoring Ethylene oxide (EtO) critical role in the medical device industry.
Medical Dynamometer Market Size
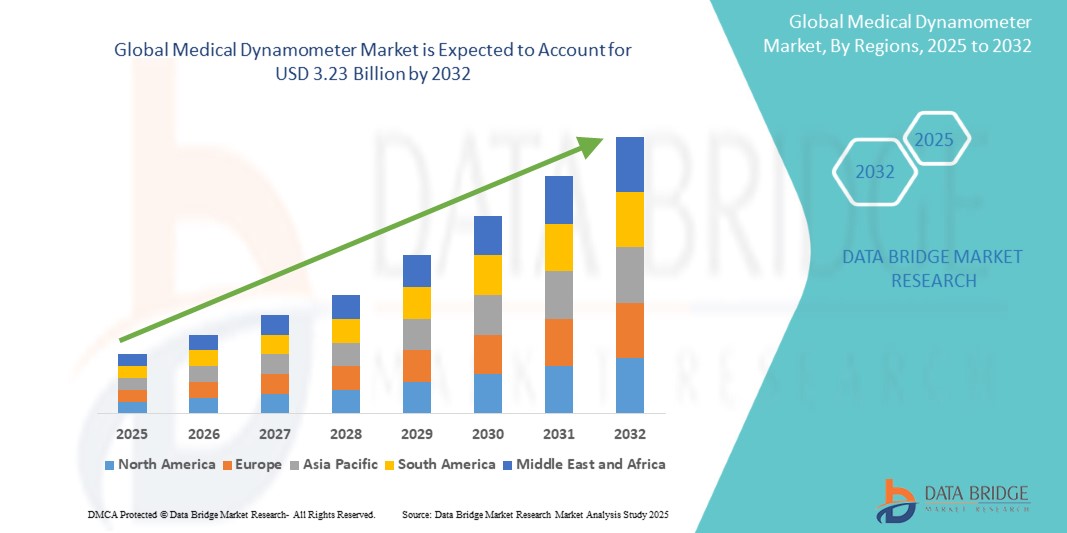
The global medical device ethylene oxide sterilization market size was valued at USD 2.18 billion in 2024 and is projected to reach USD 3.23 billion by 2032, with a CAGR of 5.05 % during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Medical Dynamometer Market Segmentation
|
Attributes |
Medical Dynamometer Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Sterigenics U.S., LLC – A Sotera Health company, STERIS, 3M , Azbil Telstar, SLU, Getinge AB, Cosmed Group , H.W.Andersen Products Ltd., Ionisos, Labtron Equipment Ltd., ETC , Inox Torres Group S.L. ,RSD ENGINEERING ,Life Science Outsourcing, Inc. ,TEKNOMAR LTD., sterilization-baltics.com., DE LAMA S.P.A ,Hubei CFULL Medical Technology Co., Ltd., INSTECH SYSTEMS, ERNA Ltd. Co., Kaustubh Enterprise, Galbino Technology Inc., SOLSTEO, Steridium, Sterility Equipment India Private Limited, HANGZHOU BOCON MECHANICAL and ELECTRICAL EQUIPMENT CO., LTD., among others |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Medical Dynamometer Market Definition
Medical device ethylene oxide sterilization refers to the process of using ethylene oxide gas to sterilize medical devices and equipment. This method is particularly suitable for items that are heat or moisture sensitive, such as plastic materials, electronic components, and certain types of packaging. Ethylene oxide effectively penetrates packaging and complex geometries, ensuring thorough disinfection without compromising the integrity of the sterilized items. This sterilization technique is widely used across various industries including healthcare, pharmaceuticals, and food production due to its versatility and effectiveness in eliminating microorganisms. It plays a critical role in ensuring the safety and efficacy of medical devices and equipment used in patient care, surgical procedures, and diagnostic applications.
Medical Dynamometer Market Dynamics
Drivers
- The Essential Role Of Ethylene Oxide In Sterilization Process
Ethylene oxide (EtO) sterilization is essential in the global medical device market due to its compatibility with a wide range of heat-sensitive and delicate devices. This method effectively sterilizes medical instruments such as catheters, pacemakers, and single-use plastics without damaging the materials or compromising their performance. Its ability to penetrate complex devices and treat packaged materials makes it a preferred option for many medical device manufacturers. Ethylene oxide (EtO) sterilization's comprehensive effectiveness against a broad spectrum of microorganisms, including highly resistant bacteria, significantly ensures patient safety and infection control during medical procedures. As the demand for advanced medical devices grows globally, market players benefit from the reliability and versatility of Ethylene oxide (EtO) sterilization, driving market growth and innovation. Ethylene oxide (EtO) ability to thoroughly sterilize complex devices without damaging them has made it an indispensable part of medical device manufacturing. This flexibility, combined with Ethylene oxide (EtO) broad-spectrum antimicrobial effectiveness, drives market growth by meeting healthcare providers' stringent safety and efficacy demands worldwide. As a result, market players continue to invest in Ethylene oxide (EtO) sterilization technologies and services, capitalizing on its proven reliability and safety.
Opportunities
- Technological Advancements Contribute To The Efficiency And Safety In Sterilization
The continuous development of technology for sterilization in the Ethylene Oxide (EO) sterilization market has created numerous opportunities for the industry. These technologies have enabled the development of more efficient and productive ethylene oxide sterilization systems, which can process a larger volume of products in a shorter time frame. This increased efficiency has led to cost savings and reduced production times, making it more attractive for manufacturers to use ethylene oxide sterilization. Advancements in technology have enhanced safety and containment measures, including improved ventilation systems, automated monitoring, and advanced control systems. These features have reduced the risk of accidents and exposure to hazardous chemicals, making ethylene oxide sterilization a more viable option for companies.
Restraints/Challenges
- High Initial Investment In Ethylene Oxide Sterilization Facilities
Building and installing an ETO sterilization facility requires a significant investment. The cost of equipment, building design, and ventilation systems can be substantial, making it a barrier to entry for new players. ETO sterilization facility is also expensive due to the costs of energy, labor, and maintenance. The high cost of ETO itself and the need for complex ventilation systems add to the overall operating expenses. Moreover, operating an ETO sterilization facility requires specialized expertise and training. This includes understanding the chemistry of ETO, the proper handling and storage of the gas, and the safe operation of the equipment. The high cost of training and retaining qualified personnel can be a significant challenge for companies.
- Diverse Sterilization Methods In The Medical Device Industry
The availability of various sterilization methods poses a significant problem for companies in the medical device sterilization market. Different healthcare providers and manufacturers require different sterilization solutions for various medical devices, leading to competition among methods such as ethylene oxide (EtO), steam sterilization, gamma radiation, and electron beam irradiation. Each method has its own advantages and limitations in terms of material compatibility, effectiveness, cost, cycle times, and regulatory compliance. Market players must either invest in multiple sterilization technologies or specialize in one approach to meet diverse customer needs. This can result in higher operational costs and the need for specialized expertise. Furthermore, as the industry changes, there may be shifts in preferences and regulatory requirements, making strategic planning more complex for market players.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Medical Dynamometer Market Scope
The market is segmented on the basis of type, material sterilized, and end user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Services
- Equipment
Material Sterilized
- Polymers
- Metals
- Others
End User
- Medical Device Manufacturers
- Hospitals
- Ambulatory Surgical Centers
- Others
Medical Dynamometer Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type, material sterilized, and end user as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to dominate the global medical device ethylene oxide sterilization market expected to dominate the market its advanced healthcare infrastructure, robust regulatory framework, and significant investment in research and development. U.S. is expected to dominate the North America market due to its advanced healthcare infrastructure, extensive medical device manufacturing industry, and stringent regulatory standards ensuring high-quality sterilization processes. China is expected to dominate in the Asia-Pacific region due to its large-scale manufacturing capabilities, expanding healthcare sector, and growing medical device industry. Gemany is expected to dominate in the Europe region due to its its advanced healthcare infrastructure, strong medical device industry, and technological innovations in sterilization processes.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Medical Dynamometer Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Medical Dynamometer Market Leaders Operating in the Market Are:
- Sterigenics U.S., LLC – A Sotera Health company
- STERIS
- 3M
- Azbil Telstar, SLU
- Getinge AB
- Cosmed Group
- H.W. Andersen Products Ltd.
- Ionisos
- Labtron Equipment Ltd.
- ETC
- Inox Torres Group S.L.
- RSD ENGINEERING
- Life Science Outsourcing, Inc.
- TEKNOMAR LTD.
- sterilization-baltics.com
- DE LAMA S.P.A
- Hubei CFULL Medical Technology Co., Ltd.
- INSTECH SYSTEMS
- ERNA Ltd. Co.
- Kaustubh Enterprise
- Galbino Technology Inc.
- SOLSTEO
- Steridium
- Sterility Equipment India Private Limited
- HANGZHOU BOCON MECHANICAL and ELECTRICAL EQUIPMENT CO., LTD.
Latest Developments in Medical Dynamometer Market
- In February 2021, 3M has launched 3M Clean & Protect Certified Badge Program. It is a new comprehensive system for cleaning, monitoring and protecting facilities. This strategy anticipated to strengthen the company footprints in the market and accelerate its growth trajectory particularly in the long term.
- In January 2021, STERIS announced that it has completed the acquisition of Cantel Medical. Through this acquisition, STERIS complement and extend its product and service offerings, global reach and customers. This acquisition has increased the sales and demands of the product and boosted the growth.
- In November 2020, Solsteo launched a new ethylene oxide sterilizer. This launch integrated pre-conditioning, sterilization and aeration in one cycle, reduces EO exposure risks, and makes it easier to operate.
- In October 2020, Getinge AB announced the launch of cost-efficient sterilization with Getinge’s new steam sterilizer Solsus 66. The new sterilizer is easy to use and install and is energy efficient. This product launch has broadened the product portfolio of the company and boosted the market growth.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.