Global Intra-Uterine Contraceptive Devices Market, By Type (Hormonal Intrauterine Device, Copper Intrauterine Device), Product Type (Mirena, Skyla, Paragard, Essure, Levosert, Others), End User (Hospitals, Gynaecology Clinics, Community Health Care Centers, Others) – Industry Trends and Forecast to 2031.
Intra-Uterine Contraceptive Devices Market Analysis
In the recent years, more than a half of the pregnancies are unwanted, unintended and mistimed. Various governments and non-profit organizations are increasing initiatives to promote the use of IUDs. Various awareness programmes are being organized to educate people regarding birth control measures and family planning.
Intra-Uterine Contraceptive Devices Market Size
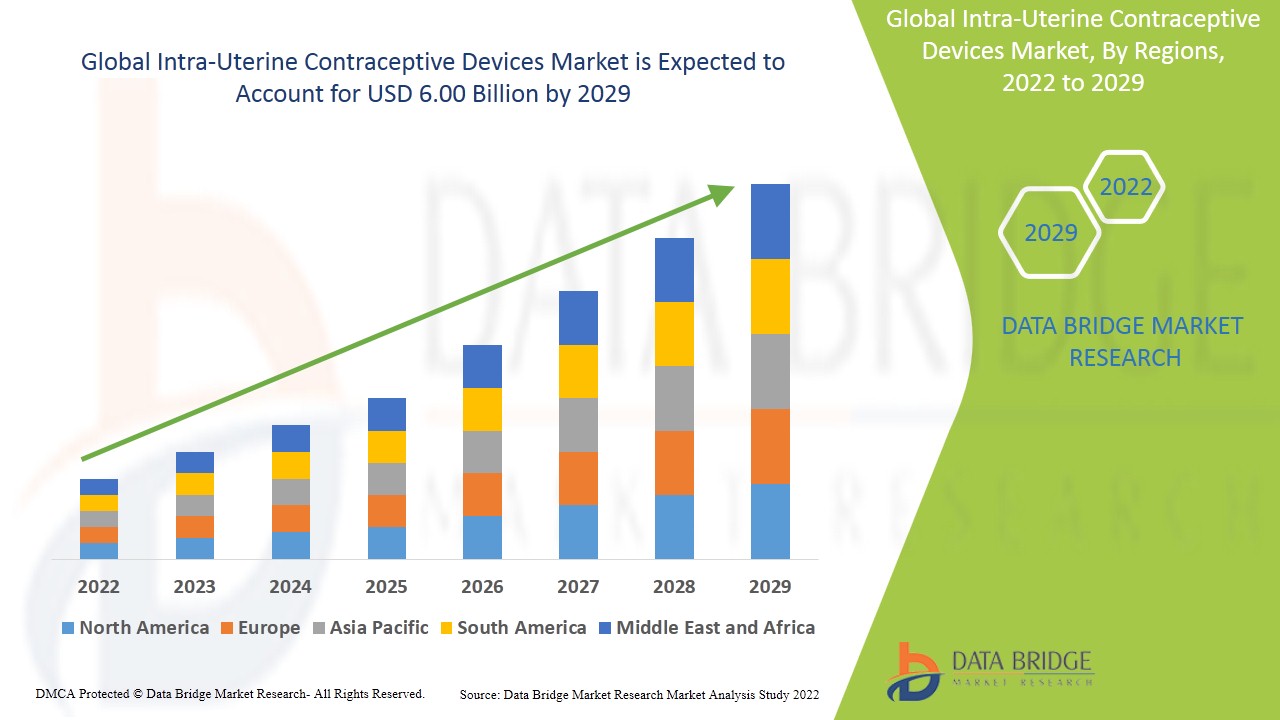
Global Intra-Uterine Contraceptive Devices Market size was valued at USD 4.48 billion in 2023 and is projected to reach USD 6.47 billion by 2031, with a CAGR of 4.70% during the forecast period of 2024 to 2031.
Report Scope and Market Segmentation
|
Attributes
|
Intra-Uterine Contraceptive Devices Key Market Insights
|
|
Segmentation
|
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
|
|
Key Market Players
|
Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd.(Israel), Sanofi (France), Novartis AG (Switzerland), Bayer AG (Germany), Lilly (U.S.), Merck & Co., Inc. (U.S.), AstraZeneca (U.K.), Johnson & Johnson Private Limited (U.S.), Biocon (India), Boehringer Ingelheim International GmbH. (Germany), Medtronic (Ireland), Ypsomed AG (Switzerland), Julphar (UAE), Albireo Pharma, Inc. (U.S.). CeQur Simplicity (Switzerland), BD (U.S.), B. Braun SE (Germany), Novo Nordisk A/S (Denmark), WOCKHARDT (India)
|
|
Market Opportunities
|
|
Intra-Uterine Contraceptive Devices Market Definition
Intrauterine devices (IUDs) are defined as copper birth control or small T-shaped plastic devices that are inserted into the uterus. These devices are known to prevent pregnancy as it stops sperm from reaching and fertilizing the eggs. These devices are being highly deployed as they are considered safe, long-acting, effective and eliminating the need for other contraceptives.
Intra-Uterine Contraceptive Devices Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints and challenges. All of this is discussed in detail as below:
- Rise in Awareness
The rise in awareness regarding the birth control measures and family planning across the globe acts as one of the major factors driving the growth of intra-uterine contraceptive devices market.
- Initiatives by Government
The increase in the initiatives by several governments and non-profit organizations to promote the use of IUDs accelerate the market growth.
- Affordable Care Act (ACA)
The repealing Affordable Care Act (ACA) in the United States encouraging large section of women to choose for long-acting reversible contraception (LARC) owing to the lack of access to abortion further influence the market.
Opportunities
Furthermore, technological innovations leading to effective contraception and fewer side effects extend profitable opportunities to the market players in the forecast period of 2024 to 2031.
Restraints/Challenges
On the other hand, common risks associated with intrauterine contraceptive devices such as, infections, damage to the uterus and severe cramping, among others are expected to obstruct market growth. Also, stringent regulations and reimbursement policies are projected to challenge the intra-uterine contraceptive devices market in the forecast period of 2024-2031.
This intra-uterine contraceptive devices market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on intra-uterine contraceptive devices market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Intra-Uterine Contraceptive Devices Market
The COVID-19 pandemic had an impact on the health systems across the globe, disrupting access to family planning information and services, also affecting intra-uterine contraceptive devices market. The need for family planning remains the same despite this disruption as family planning is critical health care for women. Governments were working with global partners in order to ensure family planning remains an essential element of the global health agenda during pandemic, as well as post-pandemic.
Intra-Uterine Contraceptive Devices Market Scope
The intra-uterine contraceptive devices market is segmented on the basis of type, product type and end user. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Hormonal Intrauterine Device
- Copper Intrauterine Device
Product Type
- Mirena
- Skyla
- Paragard
- Essure
- Levosert
- Others
End User
- Hospitals
- Gynecology Clinics
- Community Health Care Centers
- Others
Intra-Uterine Contraceptive Devices Market Regional Analysis
The intra-uterine contraceptive devices market is analyzed and market size insights and trends are provided by country, type, product type and end user as referenced above.
The countries covered in the intra-uterine contraceptive devices market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the intra-uterine contraceptive devices market because of the technological advancements in intrauterine contraceptive devices within the region.
Asia-Pacific (APAC) is expected to witness significant growth during the forecast period of 2024 to 2031 due to the rise in awareness among the woman population in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The intra-uterine contraceptive devices market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for intra-uterine contraceptive devices market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the intra-uterine contraceptive devices market. The data is available for historic period 2010-2020.
Intra-Uterine Contraceptive Devices Market Share
The intra-uterine contraceptive devices market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to intra-uterine contraceptive devices market.
Intra-Uterine Contraceptive Devices Market Leaders Operating in the Market Are:
- Teva Pharmaceutical Industries Ltd. (Jerusalem)
- Bayer (Germany)
- EUROGINE (Spain)
- CooperSurgical Inc. (US)
- Pfizer Inc. (US)
- HLL Lifecare Limited (Thiruvananthapuram)
- OCON Medical Ltd. (Israel)
- Prosan International BV (Netherlands)
- Melbea Innovations (Hungary)
- Allergan, Merck & Co., Inc. (US)
- DKT International (US)
- Medisafe Distribution Inc. (Canada)
- Medicines360 (US)
- Pregna International Limited (India)
- Egemen International (Turkey)
Latest Developments in Intra-Uterine Contraceptive Devices Market
- In June’2021, Sebela Pharmaceuticals Inc., collaborated with PRA Health Sciences to conduct a phase III clinical trial to assess LevoCept. The product is a long-acting reversible intrauterine system for contraceptive safety, tolerability and efficacy. The trial is going to be completed
SKU-