Global Ibuprofen Api Market
Market Size in USD Million
CAGR :
% 
 USD
470.72 Million
USD
690.19 Million
2024
2032
USD
470.72 Million
USD
690.19 Million
2024
2032
| 2025 –2032 | |
| USD 470.72 Million | |
| USD 690.19 Million | |
|
|
|
|
Ibuprofen API Market Size
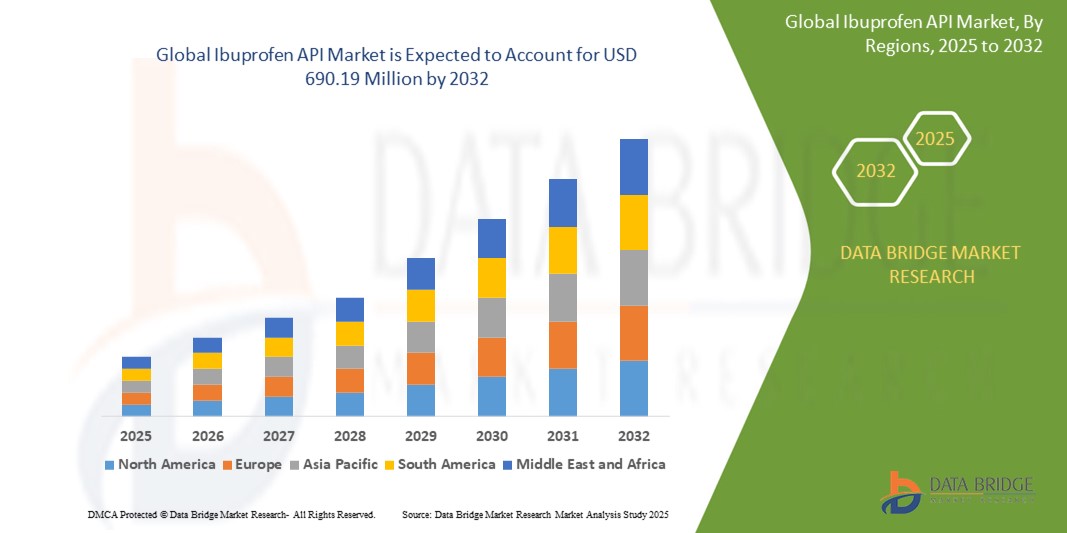
- The global ibuprofen API market size was valued at USD 470.72 million in 2024 and is expected to reach USD 690.19 million by 2032, at a CAGR of 4.90% during the forecast period
- The market growth is largely fueled by the rising global demand for over-the-counter (OTC) pain relief medications and the increasing prevalence of conditions such as headaches, arthritis, and musculoskeletal disorders, which have elevated the need for effective non-prescription analgesics such as ibuprofen
- Furthermore, advancements in API manufacturing technologies, expansion of pharmaceutical production capacities in emerging economies, and regulatory approvals across major global markets are accelerating the uptake of ibuprofen APIs, thereby significantly boosting the industry's growth
Ibuprofen API Market Analysis
- Ibuprofen API is a widely used nonsteroidal anti-inflammatory drug (NSAID) ingredient formulated in analgesic and antipyretic medications to relieve pain, reduce inflammation, and lower fever. It is a key component in both branded and generic drug formulations across oral and topical delivery forms
- The growing adoption of generic drugs, increasing self-medication trends, and the rising burden of chronic and acute pain conditions are primary drivers of market expansion. In addition, global pharmaceutical manufacturers are focusing on cost-effective and large-scale API production to meet rising demand across regulated and non-regulated markets
- North America dominated the ibuprofen API market with a share of over 40% in 2024, due to high consumption of OTC analgesics and a well-established pharmaceutical manufacturing infrastructure
- Asia-Pacific is expected to be the fastest growing region in the ibuprofen API market with a share of during the forecast period due to rising healthcare access, expanding pharmaceutical exports, and favorable cost dynamics in countries such as India and China
- Standard ibuprofen segment dominated the market with a market share of 69% in 2024, due to its widespread use in over-the-counter (OTC) medications targeting common ailments such as headaches, menstrual pain, and muscle aches. Its consistent efficacy, established regulatory approval, and cost-effectiveness have made it a mainstay ingredient across global pharmaceutical formulations. The market for standard ibuprofen remains robust due to its continued inclusion in combination drugs and high demand from generic drug manufacturers
Report Scope and Ibuprofen API Market Segmentation
|
Attributes |
Ibuprofen API Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Ibuprofen API Market Trends
“Rising Demand for Cost-Effective Medications”
- The global Ibuprofen API market is experiencing strong momentum as healthcare systems and consumers increasingly seek affordable and accessible pain relief options, particularly in regions where out-of-pocket healthcare spending is high
- For instance, IOL Chemicals and Pharmaceuticals Ltd. has ramped up its ibuprofen API production capacity in India, leveraging large-scale manufacturing efficiencies to supply competitively priced APIs to both domestic and international pharmaceutical companies
- The growing presence of generic drug manufacturers, especially in Asia, is intensifying price competition, compelling API producers to innovate and optimize their processes to maintain profitability while meeting the rising demand
- Adoption of advanced production technologies, such as continuous manufacturing and green chemistry, is enabling ibuprofen API producers to reduce operational costs, minimize environmental impact, and ensure consistent product quality
- The increasing proportion of elderly populations worldwide is leading to a higher prevalence of chronic pain and inflammatory conditions, which in turn is fueling sustained demand for ibuprofen-based medications across both OTC and prescription markets
- Regulatory authorities and governments are supporting initiatives to improve access to essential medicines, further driving the market for cost-effective ibuprofen APIs and encouraging local manufacturing in emerging economies
Ibuprofen API Market Dynamics
Driver
“Increasing Demand for Pain Management”
- The rising incidence of chronic pain, musculoskeletal disorders, and inflammatory diseases is a major factor propelling the demand for ibuprofen APIs, as healthcare providers and patients seek effective and widely available pain management solutions
- For instance, BASF SE has reported a significant uptick in global demand for its ibuprofen API, with a notable surge in orders from OTC drug manufacturers in North America and Europe, reflecting the growing reliance on ibuprofen for everyday pain relief
- The trend toward self-medication, supported by the easy accessibility of OTC ibuprofen products, is contributing to higher consumption rates, especially in developed markets where consumers are proactive about managing minor health issues
- Expansion of pharmaceutical manufacturing infrastructure in Asia-Pacific, particularly in India and China, is increasing the global supply of ibuprofen APIs and enabling broader distribution of ibuprofen-based medications
- Ibuprofen’s strong safety and efficacy profile, combined with its long-standing acceptance among healthcare professionals, is reinforcing its position as a preferred choice for both acute and chronic pain management in diverse patient populations
Restraint/Challenge
“Fluctuating Raw Material Prices”
- Volatility in the prices of key raw materials, such as isobutylbenzene and acetyl chloride, poses a significant challenge for ibuprofen API manufacturers, impacting their cost structures and ability to offer competitive pricing
- For instance, Shandong Xinhua Pharmaceutical Co., Ltd. faced operational challenges and margin pressures in 2023 due to sudden spikes in raw material costs, underscoring the vulnerability of API producers to global supply chain disruptions and commodity price swings
- Meeting stringent regulatory requirements from agencies such as the US FDA and EMA requires substantial investment in quality control, documentation, and compliance processes, which can increase the time and cost involved in bringing APIs to market
- Intense price competition from generic drug manufacturers forces API producers to operate with narrow profit margins, making it challenging to absorb fluctuations in production costs without compromising on quality or supply reliability
- Growing emphasis on environmental sustainability and stricter emission norms are raising operational costs for API manufacturers, particularly in regions where regulatory authorities are tightening environmental compliance standards
Ibuprofen API Market Scope
The market is segmented on the basis of type, application, and end-user.
- By Type
On the basis of type, the Ibuprofen API market is segmented into standard ibuprofen and high potency ibuprofen. The standard ibuprofen segment dominated the largest market revenue share of 69% in 2024, attributed to its widespread use in over-the-counter (OTC) medications targeting common ailments such as headaches, menstrual pain, and muscle aches. Its consistent efficacy, established regulatory approval, and cost-effectiveness have made it a mainstay ingredient across global pharmaceutical formulations. The market for standard ibuprofen remains robust due to its continued inclusion in combination drugs and high demand from generic drug manufacturers.
The high potency ibuprofen segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by rising clinical preference for lower-dosage, high-efficacy formulations in chronic conditions. Demand is increasing across prescription drug categories, particularly for treating conditions requiring prolonged anti-inflammatory therapy such as rheumatoid arthritis. This segment is also benefiting from innovations in drug delivery systems and enhanced bioavailability, which are encouraging broader adoption by pharmaceutical developers.
- By Application
On the basis of application, the market is segmented into headache, arthritis, and others. The headache segment accounted for the largest share of revenue in 2024, driven by the high global prevalence of tension-type and migraine headaches and the widespread use of ibuprofen as a first-line analgesic. Ease of accessibility and affordability have made ibuprofen a preferred treatment option in both developing and developed healthcare systems.
The arthritis segment is projected to register the fastest growth from 2025 to 2032, propelled by the growing geriatric population and rising incidence of osteoarthritis and rheumatoid arthritis. Ibuprofen’s anti-inflammatory properties, combined with increased adoption of long-term pain management therapies, are encouraging healthcare professionals to recommend ibuprofen-based treatment. In addition, advancements in sustained-release ibuprofen formulations are expanding its utility in chronic care settings.
- By End User
On the basis of end user, the Ibuprofen API market is segmented into CROs and CMOs and pharmaceutical and biopharmaceutical companies. The pharmaceutical and biopharmaceutical companies segment held the largest revenue share in 2024 due to the consistent and large-scale demand for ibuprofen APIs in the production of branded and generic drugs. These companies maintain strategic API procurement and in-house formulation processes to meet domestic and international regulatory standards, thereby sustaining high-volume consumption.
The CROs and CMOs segment is expected to witness the highest CAGR from 2025 to 2032, driven by increasing outsourcing trends among pharma giants looking to optimize costs and focus on core R&D. CMOs are gaining preference for their scalability, technical expertise, and faster time-to-market capabilities, especially for high-volume API production. Moreover, growing reliance on CROs for preclinical studies and formulation development is expanding demand across this segment.
Ibuprofen API Market Regional Analysis
- North America dominated the ibuprofen API market with the largest revenue share of over 40% in 2024, driven by high consumption of OTC analgesics and a well-established pharmaceutical manufacturing infrastructure
- The region's advanced healthcare system, coupled with strong consumer awareness regarding self-medication for pain relief, has sustained consistent demand for ibuprofen-based formulations
- The growing burden of lifestyle-related disorders, widespread use of anti-inflammatory drugs, and the presence of leading pharmaceutical companies continue to support market growth in both the U.S. and Canada
U.S. Ibuprofen API Market Insight
The U.S. Ibuprofen API market captured the largest revenue share in 2024 within North America, fueled by significant OTC drug consumption and a strong focus on generic drug production. A high incidence of conditions such as headaches, arthritis, and muscle pain is contributing to sustained demand. Moreover, the presence of major contract manufacturers and FDA-approved facilities has positioned the U.S. as a key production and export hub for ibuprofen APIs.
Europe Ibuprofen API Market Insight
The Europe Ibuprofen API market is projected to expand at a notable CAGR over the forecast period, supported by the region's growing preference for affordable generics and non-prescription medications. Increasing regulatory emphasis on quality standards and pharmacovigilance, alongside demand for chronic pain management solutions, is boosting ibuprofen API consumption. The region’s strong R&D ecosystem and focus on sustainable API sourcing are also contributing to long-term market stability.
U.K. Ibuprofen API Market Insight
The U.K. Ibuprofen API market is expected to grow at a steady CAGR, driven by rising self-care trends and the widespread use of OTC medications. National healthcare policies promoting the use of generics and the country’s strategic role in pharmaceutical trade post-Brexit are influencing local API procurement. Furthermore, demand is supported by retail pharmacy chains and online platforms offering ibuprofen-based products.
Germany Ibuprofen API Market Insight
Germany’s Ibuprofen API market is forecasted to expand steadily, attributed to the country's strong pharmaceutical manufacturing base and high healthcare expenditure. Ibuprofen continues to be a key component in both monotherapy and combination drugs used across pharmacies and clinics. Environmental compliance and innovation in green chemistry are emerging as key drivers, with German manufacturers investing in sustainable API production practices.
Asia-Pacific Ibuprofen API Market Insight
The Asia-Pacific Ibuprofen API market is poised to grow at the fastest CAGR from 2025 to 2032, driven by rising healthcare access, expanding pharmaceutical exports, and favorable cost dynamics in countries such as India and China. Government support for bulk drug parks and initiatives to reduce import dependency on APIs are strengthening regional supply capabilities. Growing demand for generic pain relief solutions, coupled with rising OTC drug penetration, is further accelerating market growth.
India Ibuprofen API Market Insight
India accounted for the largest revenue share within Asia-Pacific in 2024, supported by its role as a leading global supplier of generic APIs. The country’s extensive network of USFDA- and WHO-GMP-approved manufacturing facilities, combined with government incentives under the PLI scheme for APIs, is driving production. In addition, domestic demand is surging due to the high burden of non-communicable diseases and a growing reliance on self-medication.
China Ibuprofen API Market Insight
China’s Ibuprofen API market is expanding rapidly due to the nation’s large-scale pharmaceutical production capabilities and strong export orientation. The country benefits from economies of scale, cost-effective raw material access, and integration across the drug supply chain. Rising demand for affordable pain relief medications within the domestic market, along with strategic investments in API innovation and quality compliance, are further propelling growth.
Ibuprofen API Market Share
The ibuprofen API industry is primarily led by well-established companies, including:
- BASF (Germany)
- SI Group, Inc. (U.S.)
- BIOCAUSE Inc. (U.S.)
- IOL Chemicals and Pharmaceuticals Limited (India)
- Zibo Xinhua-Perrigo Pharmaceutical Co., Ltd. (China)
- Granules India Limited (India)
- LGM Pharma (U.S.)
- Salvavidas (India)
- Taj Pharmaceuticals Limited (India)
- Dr. Reddy's Laboratories Ltd. (India)
- Sino-US Zibo Xinhua-Perrigo Pharmaceutical Co., Ltd. (China)
- Solara Active Pharma Sciences Limited (India)
- Strides Pharma Science Limited (India)
- SX Pharma (China)
Latest Developments in Global Ibuprofen API Market
- In July 2023, Granules India Limited received approval from the U.S. FDA for its Acetaminophen and Ibuprofen combination tablets, intended for treating headaches, minor arthritis pain, and toothaches. This approval enables Granules India to strengthen its product portfolio in the high-demand U.S. market, enhancing its credibility in regulated markets. The move is expected to boost ibuprofen API demand due to increased production requirements for the approved formulation
- In February 2023, Univar Solutions Brasil Ltd. entered into a distribution agreement with SI Group to expand its ibuprofen offerings in Brazil. This partnership is aimed at improving market penetration and product availability across Latin America. By reinforcing supply chain networks and local distribution capabilities, this move is likely to accelerate ibuprofen API consumption in one of the region’s growing pharmaceutical markets
- In November 2022, Solara Active Pharma Sciences Limited secured a Certificate of Suitability (CEP) from the European Directorate for the Quality of Medicines (EDQM) for its ibuprofen API produced at its Vishakhapatnam facility in Andhra Pradesh, India. This regulatory milestone opens up export opportunities to Europe, a market with stringent quality requirements, positioning Solara as a competitive global supplier and contributing to the broader expansion of the ibuprofen API market
- In March 2022, Lonza announced a USD 935 million investment in new API manufacturing facilities in Portsmouth, New Hampshire, and Visp, Switzerland, and completed a laboratory expansion at its site in Nansha, China. These infrastructure developments aim to significantly scale up API production capacity and operational capabilities across three strategic regions. The investments are expected to support growing global demand for ibuprofen and other APIs, improving supply resilience and geographic accessibility
- In August 2021, Alkem Laboratories launched a combination of ibuprofen and famotidine tablets in the U.S., targeting the treatment of symptoms related to rheumatoid arthritis and osteoarthritis. This product launch reflects the increasing need for advanced, combination-based pain management therapies and is expected to stimulate demand for ibuprofen APIs used in chronic inflammatory conditions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Global Ibuprofen Api Market, Supply Chain Analysis and Ecosystem Framework
To support market growth and help clients navigate the impact of geopolitical shifts, DBMR has integrated in-depth supply chain analysis into its Global Ibuprofen Api Market research reports. This addition empowers clients to respond effectively to global changes affecting their industries. The supply chain analysis section includes detailed insights such as Global Ibuprofen Api Market consumption and production by country, price trend analysis, the impact of tariffs and geopolitical developments, and import and export trends by country and HSN code. It also highlights major suppliers with data on production capacity and company profiles, as well as key importers and exporters. In addition to research, DBMR offers specialized supply chain consulting services backed by over a decade of experience, providing solutions like supplier discovery, supplier risk assessment, price trend analysis, impact evaluation of inflation and trade route changes, and comprehensive market trend analysis.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.