Global Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Therapeutics Market, By Age Group (Pediatrics and Adults), Diagnosis (Biopsy and Bone Marrow Aspiration, Complete Blood Count (CBC) and Differential, Presence of Philadelphia Chromosome, Spinal Tap and Cerebrospinal Fluid (CSF) Analysis, Immunophenotyping/Phenotyping, Flow Cytometry, and Polymerase Chain Reaction (PCR)), Drug Type (Existing Drugs and Pipeline Drugs), Therapy (Targeted Drugs and Immunotherapy, Chemotherapy, Radiation Therapy, Stem Cell Transplantation, and Pipeline), Cell Type (Philadelphia Chromosome, Precursor B-Cell ALL, and T-Cell ALL), Route of Administration (Oral and Parenteral) – Industry Trends and Forecast to 2030.

Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Therapeutics Market Analysis and Size
The increase in the surge in the approvals of products for the treatment is a vital factor escalating the market growth, also rise in the initiatives taken by the government and pharmaceutical organizations to spread awareness regarding the disease, increase in the advancements in technologies for treatment of disorders that have been caused by rising investments undertaken by the manufacturers and increase in the number of new product approvals are the major factors among others driving the acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market. Moreover, rise in the technological advancements and modernization in healthcare devices and rise in the research and development activities in the healthcare sector and rising emerging markets will further create new opportunities for acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market in the forecasted period of 2023 to 2030.
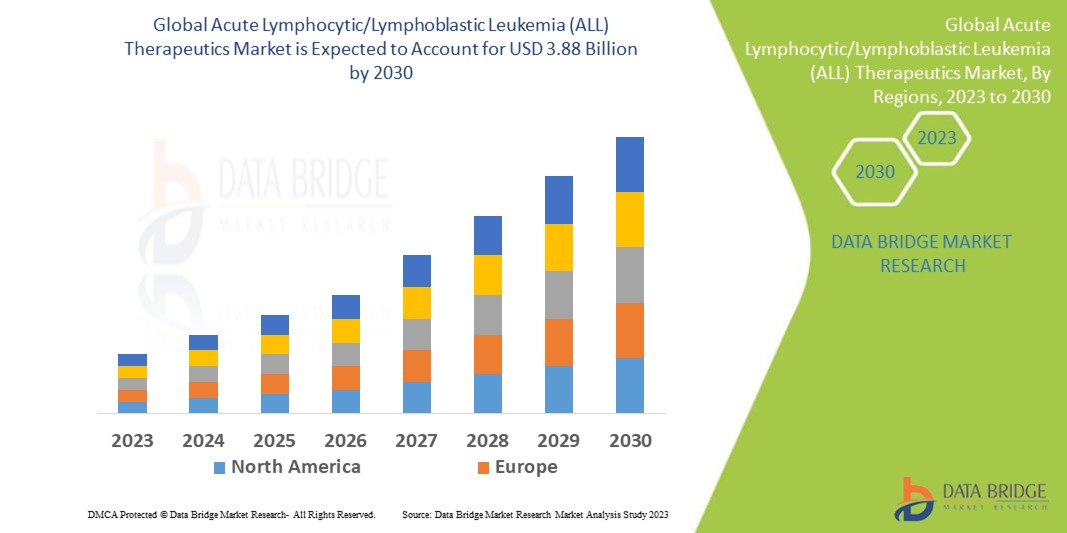
Data Bridge Market Research analyses that the global acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market which was USD 2.53 billion in 2022, would rocket up to USD 3.88 billion by 2030, and is expected to undergo a CAGR of 5.25% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Therapeutics Market Scope and Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Billion, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Age Group (Pediatrics and Adults), Diagnosis (Biopsy and Bone Marrow Aspiration, Complete Blood Count (CBC) and Differential, Presence of Philadelphia Chromosome, Spinal Tap and Cerebrospinal Fluid (CSF) Analysis, Immunophenotyping/Phenotyping, Flow Cytometry, and Polymerase Chain Reaction (PCR)), Drug Type (Existing Drugs and Pipeline Drugs), Therapy (Targeted Drugs and Immunotherapy, Chemotherapy, Radiation Therapy, Stem Cell Transplantation, and Pipeline), Cell Type (Philadelphia Chromosome, Precursor B-Cell ALL, and T-Cell ALL), Route of Administration (Oral and Parenteral)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia- Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, Rest of Middle East and Africa
|
|
Market Players Covered
|
Amgen Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), Erytech Pharma (France), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Rare Disease Therapeutics, Inc. (U.S.), Sanofi (France), Spectrum Pharmaceuticals, Inc. (U.S.), Takeda Pharmaceutical Company Limited (Japan), Genmab A/S (Denmark), Baxter (U.S.), Gilead Sciences, Inc. (U.S.), CELGENE CORPORATION (U.S.), Eisai Co., Ltd. (Japan), SymBio Pharmaceuticals Limited (Japan), Kiadis Pharma (Netherlands), OBI Pharma (Taiwan), Astellas Pharma Inc. (Japan), and Medexus Pharma, Inc. (Canada) among others
|
|
Market Opportunities
|
|
Market Definition
Acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics are the business segment dedicated to the development, production, and distribution of pharmaceutical products and treatments aimed at combating acute lymphocytic/lymphoblastic leukemia, a type of blood cancer that primarily affects white blood cells.
Global Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Therapeutics Market Dynamics
Drivers
- Increasing Aging Population
One of the drivers for the global healthcare industry is the increasing aging population. As the world's population continues to age, there is a growing demand for healthcare services, including medical treatments, long-term care, and pharmaceuticals. Older individuals often require more healthcare services due to age-related illnesses and chronic conditions, driving the overall growth of the healthcare sector. This demographic trend presents an opportunity for healthcare providers and pharmaceutical companies to cater to the healthcare needs of the elderly population
- Technological Advancements in Healthcare
Technological advancements in healthcare are a significant driver for the industry. Innovations such as telemedicine, wearable health devices, artificial intelligence (AI) in diagnostics, and robotic surgery have improved patient care, efficiency, and accuracy in diagnosis and treatment. These technologies not only enhance the patient experience but also contribute to cost savings and improved outcomes. Healthcare organizations that invest in and adopt these technologies can gain a competitive edge and provide higher-quality care to their patients
Opportunity
- Personalized Medicine and Biomarker Development
A promising opportunity in the ALL therapeutics market is the advancement of personalized medicine and the development of biomarkers. Personalized medicine involves tailoring treatment strategies to individual patients based on their genetic, genomic, and molecular profiles. In the case of ALL, identifying specific genetic mutations or biomarkers that drive the disease can enable oncologists to select the most appropriate and effective treatment for each patient
The development of biomarkers for ALL can help in early diagnosis, prognosis prediction, and treatment response monitoring. Companies that invest in research to identify relevant biomarkers and develop diagnostic tests can create valuable tools for clinicians and contribute to the growth of personalized treatment approaches
Restraint/Challenge
- Healthcare Regulatory Challenges
One major restraint in the healthcare industry is the complex and ever-changing regulatory landscape. Healthcare regulations vary from country to country and can be subject to frequent updates. Complying with these regulations requires significant resources, including legal expertise, documentation, and adherence to strict standards of care. Navigating regulatory challenges can be time-consuming and costly for healthcare providers and pharmaceutical companies, potentially slowing down the development and delivery of new treatments and services. This regulatory burden can act as a constraint on the growth and innovation within the healthcare industry
This acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth
Recent Developments
- In August 2023, Genmab A/S announced that on September 12, 2023, at 3:35 PM EDT/9:35 PM CEST, its Chief Executive Officer Jan van de Winkel, Ph.D., and Chief Financial Officer Anthony Pagano will take part in a fireside chat at the Morgan Stanley 21st Annual Global Healthcare Conference. A webcast of the event will be available, and it will feature brief opening remarks and a round of questions and answers
- In July 2023, the introduction of PERCLOT Absorbable Hemostatic Powder in the U.S. was announced by Baxter International Inc., a leader in advancing surgical innovation worldwide. For patients with intact coagulation, PERCLOT is a passive, absorbable hemostatic powder that is ready to use and intended to stop minor bleeding
Impact and Current Market Scenario of Raw Material Shortage and Shipping Delays
Data Bridge Market Research offers a high-level analysis of the market and delivers information by keeping in account the impact and current market environment of raw material shortage and shipping delays. This translates into assessing strategic possibilities, creating effective action plans, and assisting businesses in making important decisions.
Apart from the standard report, we also offer in-depth analysis of the procurement level from forecasted shipping delays, distributor mapping by region, commodity analysis, production analysis, price mapping trends, sourcing, category performance analysis, supply chain risk management solutions, advanced benchmarking, and other services for procurement and strategic support.
Global Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Therapeutics Market Scope
The global acute lymphocytic/lymphoblastic leukemia (ALL) Therapeutics Market is segmented on the basis of age group, diagnosis, drug type, therapy, cell type, and route of administration. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Age Group
- Pediatrics
- Adults
Diagnosis
- Biopsy and Bone Marrow Aspiration
- Complete Blood Count (CBC) and Differential
- Presence of Philadelphia Chromosome
- Spinal Tap and Cerebrospinal Fluid (CSF) Analysis
- Immunophenotyping/Phenotyping
- Flow Cytometry
- Polymerase Chain Reaction (PCR)
Drug Type
- Existing Drugs
- Pipeline Drugs
Therapy
- Targeted Drugs and Immunotherapy
- Chemotherapy
- Radiation Therapy
- Stem Cell Transplantation
- Pipeline
Cell Type
- Philadelphia Chromosome
- Precursor B-Cell ALL
- T-Cell ALL
Route of Administration
- Oral
- Parenteral
Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Therapeutics Market Regional Analysis/Insights
The acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market is analyzed and market size insights and trends are provided by age group, diagnosis, drug type, therapy, cell type and route of administration as referenced above.
The countries covered in the global acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market report are U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia- Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, Rest of Middle East and Africa
North America dominates and register the highest growth rate in the acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market due to rise in the surge in the approvals of products for the treatment and rise in the initiatives taken by the government and pharmaceutical organizations to spread awareness regarding the disease in this region.
Asia-Pacific is the expected region in terms of growth in acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market due to rise in the prevalence of acute lymphocytic/lymphoblastic leukemia (ALL) in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and up-stream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the Market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Therapeutics Market Share Analysis
The acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the company’s focus related to acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market.
Some of the major players operating in the acute lymphocytic/lymphoblastic leukemia (ALL) therapeutics market are:
- Amgen Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Erytech Pharma (France)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Rare Disease Therapeutics, Inc. (U.S.)
- Sanofi (France)
- Spectrum Pharmaceuticals, Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Genmab A/S (Denmark)
- Baxter (U.S.)
- Gilead Sciences, Inc. (U.S.)
- CELGENE CORPORATION (U.S.)
- Eisai Co., Ltd. (Japan)
- SymBio Pharmaceuticals Limited (Japan)
- Kiadis Pharma (Netherlands)
- OBI Pharma (Taiwan)
- Astellas Pharma Inc. (Japan)
- Medexus Pharma, Inc. (Canada)
SKU-

