Europe Medical Device Testing Market, By Service Type (Testing Services, Inspection Service and Certification Services), Testing Type (Physical Testing, Chemical/Biological Testing, Cybersecurity Testing, Microbiology and Sterility Testing and Others), Phase (Preclinical and Clinical), Sourcing Type (In-House and Outsourced), Device Class (Class I, Class II and Class III), Product (Active Implant Medical Device, Active Medical Device, Non-Active Medical Device, In-vitro Diagnostics Medical Device, Ophthalmic Medical Device, Orthopedic and Dental Medical Device, Vascular Medical Device and Others) Industry Trends and Forecast to 2029
Europe Medical Device Testing Market Analysis and Insights
Medical device testing is the process of demonstrating that the device is reliably and safely perform in use. In new product development, extensive design validation testing is applied. This includes performance testing, toxicity, chemical analysis, and sometimes human factors or clinical testing. Ongoing quality assurance testing is generally more limited. This usually includes dimensional checks, some functional tests, and packaging verification. Various types of medical testing services are available there in the market, such as inspection services, certification services, and others
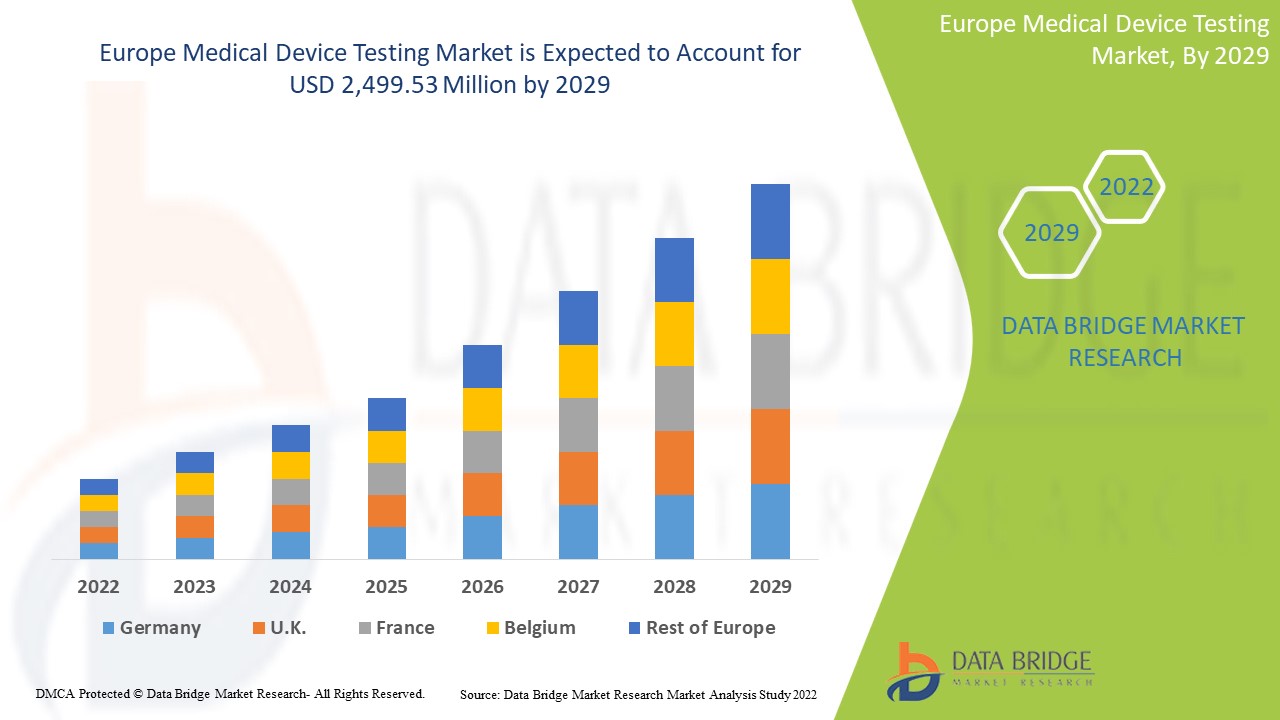
Europe medical device testing market is expected to grow in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 9.8% in the forecast period of 2022 to 2029 and is expected to reach USD 2,499.53 million by 2029 from USD 1,226.46 million in 2021.
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2019-2014)
|
|
Quantitative Units
|
Revenue in USD Million, Pricing in USD
|
|
Segments Covered
|
By Service Type (Testing Services, Inspection Service and Certification Services), Testing Type (Physical Testing, Chemical/Biological Testing, Cybersecurity Testing, Microbiology and Sterility Testing and Others), Phase (Preclinical and Clinical), Sourcing Type (In-House and Outsourced), Device Class (Class I, Class II and Class III), Product (Active Implant Medical Device, Active Medical Device, Non-Active Medical Device, In-vitro Diagnostics Medical Device, Ophthalmic Medical Device, Orthopedic and Dental Medical Device, Vascular Medical Device and Others)
|
|
Countries Covered
|
Germany, France,U.K., Italy, Spain, Netherlands, Russia, Switzerland, Turkey, Belgium and Rest of Europe
|
|
Market Players Covered
|
Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, UL LLC, North American Science Associates, LLC, Medistri SA, WuXi AppTec, NSF, Labcorp, Eurofins Scientific, Nelson Laboratories, LLC- A Sotera Health company, ITC ZLIN, Element Materials Technology, EndoLab Mechanical Engineering GmbH, Hohenstein, Medical Engineering Technologies Ltd., Cigniti, IMR Test Labs and among others
|
Market Definition
Medical device testing is the process of demonstrating that the device is reliably and safely perform in use. In new product development, extensive design validation testing is applied. This includes performance testing, toxicity and chemical analysis, and sometimes human factors or even clinical testing. Ongoing quality assurance testing is generally more limited. This usually include dimensional checks, some functional tests, and packaging verification. Various types of medical testing services are available there in the market such as inspection services, certification services and among others
Medical Device Testing Market Dynamics
Drivers
- Escalation in innovation and technologies
Acceleration in technology development in the healthcare sector has tremendously increased in last few years. Advancement in technology of medical devices supports painless and uncomplicated treatment during disease management. Moreover, innovation and up gradation in medical devices assist in the precise and rapid result of disease diagnosis. As well as innovation in medical devices also provides cost-effectiveness of technology-based therapeutic tools during disease treatment. Moreover, many government bodies and healthcare organizations are supporting the medical research centers. The primary purpose of this support is to enhance the health care innovations in Europe
- Increase in the demand for in-vitro tests
In-vitro diagnostics (IVD) are tests on samples such as blood or tissue taken from the human body. In-vitro diagnostics can detect diseases or other conditions and can be used to monitor a person’s overall health to help cure, treat, or prevent diseases. In-vitro tests are used in various disease detections such as HIV infections, malaria, hepatitis, among others. The prevalence of such diseases is rapidly increasing across European countries, leading to the increasing demand for in-vitro tests and various medical devices.
Opportunity
-
Rise in the healthcare expenditure
Healthcare expenditure has increased worldwide as people's disposable income in various countries is increasing. Moreover, to accomplish the population requirements, the government bodies and healthcare organizations are taking the initiative by accelerating healthcare expenditure. The rise in healthcare expenditure simultaneously helps healthcare settings to improve their medical device testing services over the recent years
Also, the strategic initiatives key market players take will provide structural integrity and future opportunities for the medical device testing market in the forecast period of 2022-2029.
Restraint/Challenge
- High competition in medical technology industries
However, the barriers to the local development of medical devices and the high cost of medical devices in some regions may impede the less production of medical devices hampering the market's growth. Additionally, high competition in medical technology industries and long lead time for the overseas qualification can be the challenging factors for the development of the market
This medical device testing market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Post COVID-19 Impact on Medical Device Testing Market
COVID-19 has positively affected the market. The use of medical devices has increased in those years such as MRI scanners, ventilators, and others. Hence the use of various devices has widely increased worldwide. Hence, the pandemic has affected positively on this testing market.
Recent Development
- In April 2021, TUV SUD announced that it had presented itself at Medtec LIVE to exhibit its ability to be a one-stop-shop for medical device testing. The company’s services covered testing in electrical and functional safety, cyber security and software, EMC, and biocompatibility. The experts from TUV SUD featured in the online trade show and congress program with various talks, a live hack, and an elevator pitch.
Europe Medical Device Testing Market Scope
Europe medical device testing market is segmented into service type, testing type, phase, sourcing type, device class and product. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Service Type
- Testing services
- Inspection services
- Certification services
On the basis of service type, the Europe medical device testing market is segmented into testing services, inspection services and certification services.
Testing Type
- Physical testing
- Chemical/biological testing
- Cybersecurity testing
- Microbiology and sterility testing
- Others
On the basis of testing type, the Europe medical device testing market is segmented into physical testing, chemical/biological testing, cybersecurity testing, microbiology, and sterility testing and others.
Phase
- Preclinical
- Clinical
On the basis of phase, the Europe medical device testing market is segmented into preclinical and clinical.
.Sourcing Type
- Outsourced
- In-house
On the basis of sourcing type, the Europe medical device testing market is segmented into in-house and outsourced.
Device Class
- Class I
- Class II
- Class III
On the basis of device class, the Europe medical device testing market is segmented into class I, class II and class III.
Product
- Active implant medical device
- Active medical device
- Non-active medical device
- In-vitro diagnostics medical device
- Opthalmic medical device
- Orthopedic and dental medical device
- Vascular medical device
- Others
On the basis of product, the Europe medical device testing market is segmented into active implant medical device, active medical device, non-active medical device, in-vitro diagnostics medical device, opthalmic medical device, orthopedic and dental medical device, vascular medical device and others.
Medical Device Testing Market Regional Analysis/Insights
The medical device testing market is analysed and market size insights and trends are provided by country, service type, testing type, phase, sourcing type, device class and product as referenced above.
The countries covered in the Europe region are U.K., Germany, Italy, France, Spain, Russia, Netherlands, Switzerland, Turkey, Belgium and Rest of Europe
Germany dominates the medical device testing market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the increasing demand for in-vitro tests in Europe.
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of global brands and their challenges faced due to high competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Medical Device Testing Market Share Analysis
The medical device testing market competitive landscape provides details by the competitors. Details include company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on medical device testing market.
Some of the major players operating in the medical device testing market are Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, UL LLC, North American Science Associates, LLC, Medistri SA, WuXi AppTec, NSF, Labcorp, Eurofins Scientific, Nelson Laboratories, LLC- A Sotera Health company, ITC ZLIN, Element Materials Technology, EndoLab Mechanical Engineering GmbH, Hohenstein, Medical Engineering Technologies Ltd., Cigniti, IMR Test Labs and among others.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analysed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Europe vs. Regional and Vendor Share Analysis. Please request analyst call in case of further inquiry.
SKU-

