Europe Cervical Cancer Diagnostic Market Analysis and Insights
The Europe cervical cancer diagnostic market is growing in the forecast year due to the rise in market players and the availability of various cervical cancer diagnostic products and brands. Along with this, market players are engaged in advanced cervical cancer diagnostics. The rising prevalence of cervical cancer is further expected to boost market growth. However, the stringent rules and regulations may restrain market growth in the forecast period. The various government and private collaborations, increasing R&D activities, and strategic initiatives by market players are giving opportunities to the market. However, false results in cervical cancer screening tests are projected to be a key challenge to market growth.
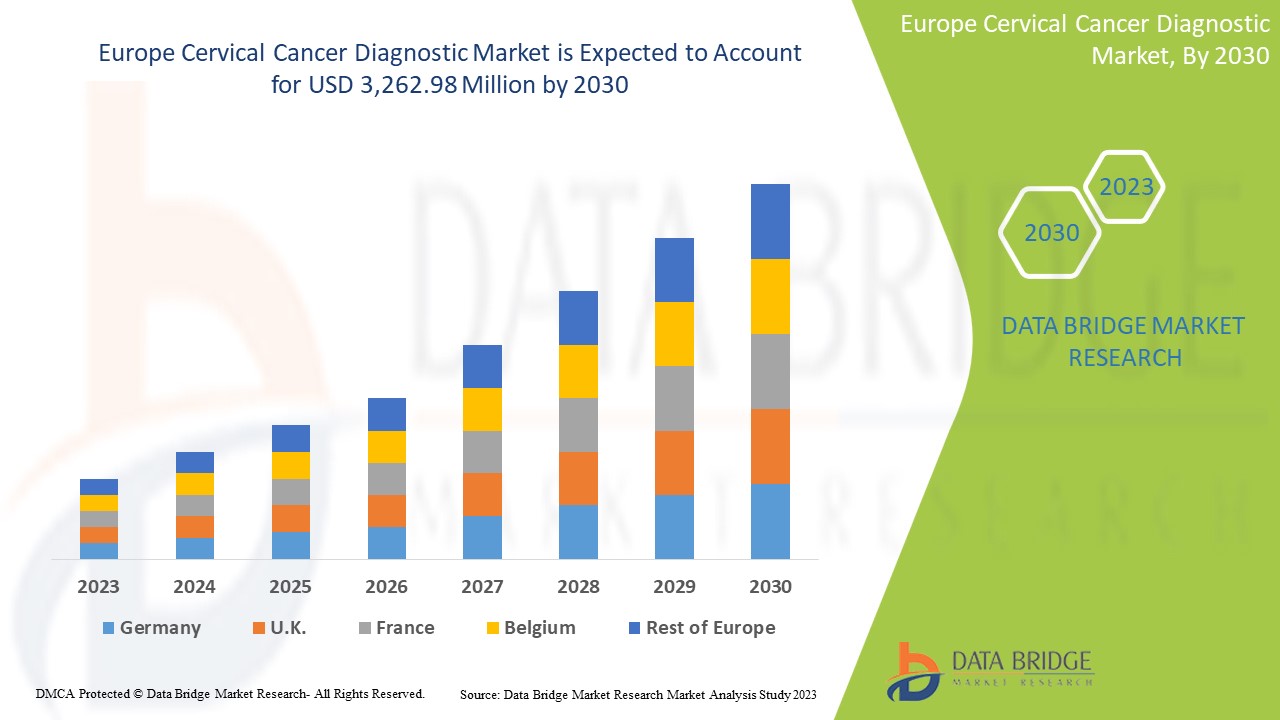
The Europe cervical cancer diagnostic market is expected to gain market growth in the forecast period of 2023 to 2030. Data Bridge Market Research analyses that the market is growing with a CAGR of 6.4% in the forecast period of 2023 to 2030 and is expected to reach USD 3,262.98 million by 2030.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customisable to 2020-2015) |
|
Quantitative Units |
Revenue in USD Million |
|
Segments Covered |
By Product Type (Imaging Test, Screening Test, Visual Examination, Cervical Biopsies, and Other Procedures), Age Group (Below 21, 21-29, 30-65, 65 and Above), Stages (Stage I, Stage II, Stage III, Stage IV), End Users (Hospitals, Diagnostic Laboratories, Specialty Clinics, Community Health Centers, Cancer Research Organization, Cancer and Radiation Therapy Centers), Distribution Channel (Direct Tender, Retail Sales, Online Sales) |
|
Countries Covered |
Germany, France, U.K., Italy, Spain, Netherlands, Russia, Denmark, Switzerland, Turkey, Rest of Europe |
|
Market Players Covered |
Siemens Healthcare GmbH, BD, F. Hoffmann-La Roche Ltd, Abbott, Hologic, Inc., Quest Diagnostics Incorporated, Bio-Rad Laboratories, Inc., QIAGEN, The Cooper Companies Inc., Seegene Inc., Sysmex Corporation, MobileODT, Zilico, Jiangsu Mole Bioscience Co. Ltd., Guided Therapeutics, Inc., GenomeMe Lab Inc., Arbor Vita Corporation, and LCM GENECT Srl |
Market Definition:
Cervical cancer is a type of cancer that develops in the cervix of the female reproductive tract. It is becoming more prevalent as the number of HPV-infected patients increases, and there is a greater emphasis on early detection and treatment, which is expected to accelerate the development of cervical cancer diagnosis. Increasing government investment in raising awareness about early cancer detection and increasing healthcare spending would also propel business growth. The most common cause of cervical cancer is HPV (Human Papillomavirus) infection. Other lifestyle choices that can raise the risk include smoking, drinking, a diet low in fruits, and vegetables, taking birth control pills, and teenage sexual encounters. Since, cervical cancer is treatable once diagnosed in an early stage, women at risk of developing the disease must undergo routine testing to identify the disease early, allowing the market to expand.
Europe Cervical Cancer Diagnostic Market Dynamics
This section deals with understanding the market drivers, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
-
Rising awareness of the early diagnosis of cervical cancer
There is an enormous range of risk factors being reported for cervical cancer. Therefore awareness about its diagnosis has increased in recent years. Various diagnostic tests are available, such as PAP (Papanicolaou) testing, human papillomavirus testing, colposcopy, cervical biopsies, and cystoscopy among others. Hence, to reduce the risk factors, early diagnosis is very important.
-
Increasing prevalence and incidence of cervical cancer
Cervical cancer is a type of cancer that develops in the female reproductive tract's cervix. The irregular development of cancer cells in the cervix tissue frequently defines cervical cancer. Adenocarcinoma or squamous cell carcinoma can develop from cervical cancer. The most common cause of cervical cancer is HPV (Human Papillomavirus) infection. Cervical cancer is classified into two types' adenocarcinoma and squamous cell carcinoma. Cervical cancer is diagnosed using a variety of advanced laboratory tests, tools, and procedures that evaluate abnormal cells and strains of the human papillomavirus (HPV).
Opportunities
-
Rising healthcare expenditure
Healthcare expenditure has increased worldwide as people's disposable income in various countries increases. Moreover, to accomplish the population requirements, government bodies and healthcare organizations are taking the initiative to accelerate healthcare expenditure. The rise in healthcare expenditure simultaneously helps healthcare settings to improve their treatment facilities on cervical cancer diagnostic as the disorder has been highly prevalent in recent years.
Also, the strategic initiatives key market players take will provide structural integrity and future opportunities for the Europe cervical cancer diagnostic market in the forecast period.
Restraints/Challenges
However, cervical cancer diagnostic drugs may have adverse side effects; it is challenging to balance the risks with the benefits of treatment due to the increasing side effects of cancer medication hampering the market demand.
Moreover, increasing approvals of HPV vaccines by regulatory authorities are expected to hinder the growth of the Europe cervical cancer diagnostic market. These developments are rapidly happening across the globe to reduce the cases of cervical cancer, which can be a restraining factor for the market.
The use of various treatment drugs across the globe is rapidly increasing, and with the increasing prevalence of cervical cancer, there is a need for timely diagnosis and treatment. At the same time, the players of the cervical cancer diagnostic manufacturers in the market have to follow certain regulations to get approval from the upper authorities for launching the product in the market. These stringent guidelines need to be followed; this is one of the most difficult tasks of all the steps. The pre-market approval of various medical drugs varies from one country to another.
Recent Development
- In August 2020, Siemens Healthcare GmbH agreed with Varian Medical Systems, Inc. for acquisition; with this acquisition, Siemens Healthcare has helped develop advanced solutions to treat cancer and strengthen its position in the healthcare industry
Europe Cervical Cancer Diagnostic Market Scope
The Europe cervical cancer diagnostic market is segmented into product type, age group, stages, end users, and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Product Type
- Imaging Test
- Screening Test
- Visual Examination
- Cervical Biopsies
- Other Procedures
On the basis of product type, the Europe cervical cancer diagnostic market is segmented into imaging test, screening test, visual examination, cervical biopsies, and other procedures.
Age Group
- Below 21
- 21-29
- 30-65
- 65 and Above
On the basis of age group, the Europe cervical cancer diagnostic market is segmented into below 21, 21-29, 30-65, and 65 and above.
Stages
- Stage I
- Stage II
- Stage III
- Stage IV
On the basis of stage, the Europe cervical cancer diagnostic market is segmented into stage I, stage II, stage III, and stage IV.
End Users
- Cancer And Radiation Therapy Centers
- Hospitals
- Specialty Clinics
- Cancer Research Organization
- Diagnostic Laboratories
- Community Health Centers
On the basis of end users, the Europe cervical cancer diagnostic market is segmented into hospitals, diagnostic laboratories, specialty clinics, community health centers, cancer research organization, and cancer and radiation therapy centers.
Distribution Channel
- Direct Tender
- Retail Sales
- Online Sales

On the basis of distribution channel, the Europe cervical cancer diagnostic market is segmented into direct tender, retail sales, and online sales.
Cervical Cancer Diagnostic Market Regional Analysis/Insights
The Europe cervical cancer diagnostic market is analysed, and market size insights and trends are provided by country, product type, age group, stages, end users, and distribution channel as referenced above.
Some countries covered in the Europe cervical cancer diagnostic market are Germany, France, U.K., Italy, Spain, Netherlands, Russia, Denmark, Switzerland, Turkey and Rest of Europe.
Germany is expected to dominate the Europe cervical cancer diagnostic market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the rise in market players and the availability of various cervical cancer diagnostic products and brands.
The country section of the report also provides individual market-impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of European brands and their challenges faced due to high competition from local and domestic brands and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Europe Cervical Cancer Diagnostic Market Share Analysis
The Europe cervical cancer diagnostic market competitive landscape provides details of the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies focus on the Europe cervical cancer diagnostic market.
Some of the major players operating in the Europe cervical cancer diagnostic market are Siemens Healthcare GmbH, BD, F. Hoffmann-La Roche Ltd, Abbott, Hologic, Inc., Quest Diagnostics Incorporated, Bio-Rad Laboratories, Inc., QIAGEN, The Cooper Companies Inc., Seegene Inc., Sysmex Corporation, MobileODT, Zilico, Jiangsu Mole Bioscience Co. Ltd., Guided Therapeutics, Inc., GenomeMe Lab Inc., Arbor Vita Corporation, and LCM GENECT Srl among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE CERVICAL CANCER DIAGNOSTIC MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES MODEL
4.3 INDUSTRY INSIGHTS:
4.3.1 CERVICAL CANCER DIAGNOSIS
4.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND INCIDENCE OF CERVICAL CANCER
6.1.2 RISING AWARENESS OF EARLY DIAGNOSIS OF CERVICAL CANCER
6.1.3 HIGH PREVALENCE OF HPV-INFECTED PATIENTS AND RISING INCIDENCE OF TEENAGE SEXUAL ENCOUNTERS
6.2 RESTRAINTS
6.2.1 DEVELOPMENT IN THE FIELD OF HPV VACCINE
6.2.2 SIDE EFFECTS OF TREATMENT DRUGS
6.3 OPPORTUNITIES
6.3.1 RISING HEALTHCARE EXPENDITURE
6.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.4 CHALLENGES
6.4.1 STRINGENT RULES AND REGULATIONS
6.4.2 FALSE RESULTS IN SCREENING TESTS AND UNAVAILABILITY OF IMPROVED HEALTHCARE INFRASTRUCTURE
7 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 SCREENING TEST
7.2.1 HPV TEST
7.2.1.1 ASSAYS
7.2.1.2 KITS/REAGENTS
7.2.2 PAP TEST
7.2.2.1 ASSAYS
7.2.2.2 KITS/REAGENTS
7.2.3 BLOOD TEST
7.3 IMAGING TEST
7.3.1 PET CT-SCAN
7.3.2 MAGNETIC RESONANCE IMAGING (MRI)
7.3.3 ULTRASOUND
7.3.4 X-RAY
7.3.5 OTHERS
7.4 CERVICAL BIOPSIES
7.4.1 LIQUID BIOPSY
7.4.2 ENDOCERVICAL CURETTAGE
7.4.3 COLPOSCOPIC BIOPSY
7.5 VISUAL EXAMINATION
7.5.1 CYSTOSCOPY
7.5.2 SIGMOIDOSCOPY
7.6 OTHER PROCEDURES
8 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP
8.1 OVERVIEW
8.2 30-65
8.3 65 AND ABOVE
8.4 21-29
8.5 BELOW 21
9 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES
9.1 OVERVIEW
9.2 STAGE I
9.3 STAGE II
9.4 STAGE III
9.5 STAGE IV
10 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER
10.1 OVERVIEW
10.2 CANCER AND RADIATION THERAPY CENTERS
10.3 HOSPITALS
10.4 SPECIALTY CLINICS
10.5 CANCER RESEARCH ORGANIZATION
10.6 DIAGNOSTIC LABORATORIES
10.7 COMMUNITY HEALTH CENTERS
11 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 RETAIL SALES
11.4 ONLINE SALES
12 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 U.K.
12.1.3 FRANCE
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 BELGIUM
12.1.7 RUSSIA
12.1.8 NETHERLANDS
12.1.9 SWITZERLAND
12.1.10 TURKEY
12.1.11 REST OF EUROPE
13 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: EUROPE
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 SIEMENS HEALTHCARE GMBH
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENT
15.2 BD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 F. HOFFMANN- LA ROCHE LTD
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 ABBOTT
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 HOLOGIC, INC.
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.6 ARBOR VITA CORPORATION
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENTS
15.7 BIO-RAD LABORATORIES, INC.
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.8 GENOMEME LAB INC.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 GUIDED THERAPEUTICS, INC
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENT
15.1 JIANGSU MOLE BIOSCIENCE CO., LTD.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENTS
15.11 LCM GENECT SRL
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 MOBILEODT
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.13 QIAGEN
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUE ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 QUEST DIAGNOSTICS INCORPORATED
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 SEEGENE INC.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENTS
15.16 SYSMEX CORPORATION
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 THE COOPER COMPANIES INC
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 ZILICO
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
List of Table
TABLE 1 CERVICAL CANCER CAN BE DIAGNOSED BY VARIOUS TESTS AND BIOPSIES, AS LISTED BELOW:
TABLE 2 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 EUROPE SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 EUROPE SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 EUROPE HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 EUROPE PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 EUROPE IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 EUROPE IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 EUROPE CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 EUROPE CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 EUROPE VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 EUROPE VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 13 EUROPE OTHER PROCEDURES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 15 EUROPE 30-65 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 EUROPE 65 AND ABOVE IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 EUROPE 21-29 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 EUROPE BELOW 21 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 20 EUROPE STAGE I IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 EUROPE STAGE II IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 EUROPE STAGE III IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 EUROPE STAGE IV IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 25 EUROPE CANCER AND RADIATION THERAPY CENTERS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 EUROPE HOSPITALS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 EUROPE SPECIALTY CLINICS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 EUROPE CANCER RESEARCH ORGANIZATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 EUROPE DIAGNOSTIC LABORATORIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 EUROPE COMMUNITY HEALTH CENTERS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 32 EUROPE DIRECT TENDER IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 EUROPE RETAIL SALES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 EUROPE ONLINE SALES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 36 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 37 EUROPE SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 38 EUROPE HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 39 EUROPE PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 40 EUROPE IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 41 EUROPE CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 42 EUROPE VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 43 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 44 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 45 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 47 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 48 GERMANY SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 49 GERMANY HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 50 GERMANY PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 GERMANY IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 52 GERMANY CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 53 GERMANY VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 54 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 55 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 56 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 57 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 58 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 59 U.K. SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 60 U.K. HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 61 U.K. PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 U.K. IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 U.K. CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 64 U.K. VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 65 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 66 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 67 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 68 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 69 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 70 FRANCE SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 71 FRANCE HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 72 FRANCE PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 73 FRANCE IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 74 FRANCE CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 FRANCE VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 77 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 78 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 79 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 80 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 ITALY SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 ITALY HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 ITALY PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 84 ITALY IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 85 ITALY CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 86 ITALY VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 87 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 88 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 89 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 90 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 91 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 92 SPAIN SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 93 SPAIN HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 94 SPAIN PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 95 SPAIN IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 SPAIN CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 SPAIN VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 99 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 100 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 101 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 102 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 103 BELGIUM SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 104 BELGIUM HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 105 BELGIUM PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 106 BELGIUM IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 107 BELGIUM CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 108 BELGIUM VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 109 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 110 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 111 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 112 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 113 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 114 RUSSIA SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 115 RUSSIA HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 RUSSIA PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 117 RUSSIA IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 118 RUSSIA CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 119 RUSSIA VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 121 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 122 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 123 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 124 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 125 NETHERLANDS SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 126 NETHERLANDS HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 127 NETHERLANDS PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 128 NETHERLANDS IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 129 NETHERLANDS CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 130 NETHERLANDS VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 131 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 132 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 133 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 134 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 135 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 136 SWITZERLAND SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 137 SWITZERLAND HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 138 SWITZERLAND PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 139 SWITZERLAND IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 140 SWITZERLAND CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 141 SWITZERLAND VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 142 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 143 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 144 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 145 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 146 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 147 TURKEY SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 148 TURKEY HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 149 TURKEY PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 150 TURKEY IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 151 TURKEY CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 152 TURKEY VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 153 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 154 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 155 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 156 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 157 REST OF EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
List of Figure
FIGURE 1 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE AND INCIDENCE OF CERVICAL CANCER ARE EXPECTED TO DRIVE THE EUROPE CERVICAL CANCER DIAGNOSTIC MARKET IN THE FORECAST PERIOD
FIGURE 12 SCREENING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE CERVICAL CANCER DIAGNOSTIC MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE CERVICAL CANCER DIAGNOSTIC MARKET
FIGURE 14 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, 2022
FIGURE 15 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP 2022
FIGURE 19 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 20 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, CAGR (2023-2030)
FIGURE 21 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, LIFELINE CURVE
FIGURE 22 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, 2022
FIGURE 23 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, 2023-2030 (USD MILLION)
FIGURE 24 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, CAGR (2023-2030)
FIGURE 25 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY STAGES, LIFELINE CURVE
FIGURE 26 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, 2022
FIGURE 27 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY END USER, LIFELINE CURVE
FIGURE 30 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 32 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: SNAPSHOT (2022)
FIGURE 35 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2022)
FIGURE 36 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2023 & 2030)
FIGURE 37 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2030)
FIGURE 38 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: PRODUCT TYPE (2023-2030)
FIGURE 39 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY SHARE 2022 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












