Asia-Pacific ADME Toxicology Testing Market, By Technology (Cell Culture, High Throughput, Molecular Imaging, Omics Technology), Product Type (Instruments, Assays and Reagents, Accessories, Software Solutions), Test (In-Vivo, In-Vitro), Method (Cellular Assay, Biochemical Assay, In-Silica, Ex-Vivo), Application (Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity, Others), End User (Biopharmaceutical Companies, Contract Research Organizations, Academic and Research Institute, Others), Distribution Channel (Direct Tender, Others) – Industry Trends and Forecast to 2031.
Asia-Pacific ADME Toxicology Testing Market Analysis and Size
As per the recent data approximately 6.2 million adults over the age of 20 are newly diagnosed with heart failure. The incidence of heart failure increases with age, and it is more common among older adults. Heart failure is a leading cause of hospitalization among people aged 65 and older. The incidence rates can vary by race and ethnicity, with some groups having a higher risk of developing heart failure. ADME toxicology testing is primarily used to perform such genetic, chemical and pharmacological tests that aid in the drug discovery process from drug design to drug trials for new diseases. This procedure involves control software, various liquid-handling devices, and other detectors that aid in the rapid identification of active compounds, genetic interactions and other biomolecular interactions.
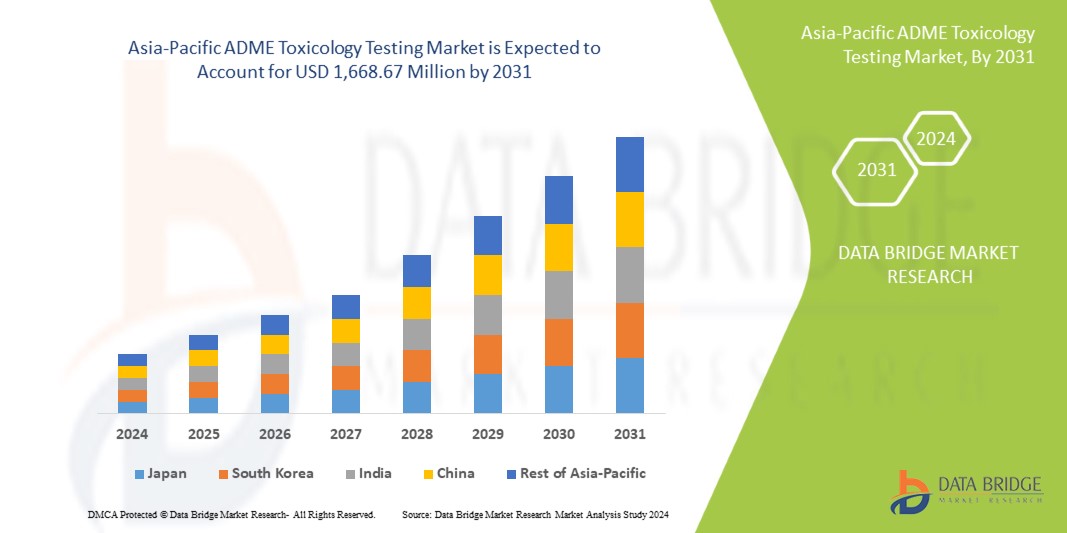
Data Bridge Market Research analyzes that the Asia-Pacific ADME toxicology testing market, which was USD 708.61 million in 2023, would rocket up to USD 1,668.67 million by 2031 and is expected to undergo a CAGR of 11.3% during the forecast period. “Cell culture” dominates the technology segment of the market due to the growing demand for better methods for treatment in patients. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Years
|
2022 (Customizable to 2016-2021)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, and Pricing in USD
|
|
Segments Covered
|
Technology (Cell Culture, High Throughput, Molecular Imaging, Omics Technology), Product Type (Instruments, Assays and Reagents, Accessories, Software Solutions), Test (In-Vivo, In-Vitro), Method (Cellular Assay, Biochemical Assay, In-Silica, Ex-Vivo), Application (Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity, Others), End User (Biopharmaceutical Companies, Contract Research Organizations, Academic and Research Institute, Others), Distribution Channel (Direct Tender, Others)
|
|
Countries Covered
|
Japan, China, India, Australia, South Korea, Singapore, Malaysia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia-Pacific
|
|
Market Players Covered
|
Promega Corporation (U.S.), Lonza (Switzerland), AAT Bioquest, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Corning Incorporated (U.S.), Charles River Laboratories (U.S.), LABCYTE INC. (U.S.), BioIVT (U.S.), Takara Bio Inc. (Japan), Agilent Technologies, Inc. (U.S.), PromoCell GmbH (Germany), Merck KGaA (Germany), and Bio-Rad Laboratories, Inc. (U.S.)
|
|
Market Opportunities
|
|
Market Definition
ADME refers to the absorption, distribution, metabolism and elimination of drugs or chemicals in and from the body. ADME testing plays a vital role in the drug development process. The testing enables pharmaceutical companies to reduce their drug discovery time, cost, and test complications.
ADME is a critical piece in drug development studies, which helps determine the viability of a drug. Toxicology study is a crucial part of drug development used to characterize a drug's toxicity profile by identifying its impact on organ structure/ functionality. The study provides critical information and knowledge used by regulatory agencies to prevent or reduce the likelihood that a disease or other negative health outcome would occur. The Food and Drug Administration has issued several guidance documents for industry such as safety testing of drug metabolites, In vitro metabolism and transporter-mediated drug-drug interaction studies, clinical drug interactions studies.
Asia-Pacific ADME Toxicology Testing Market Dynamics
Drivers
- Increasing drug development activities
The Asia-Pacific region, particularly countries like China and India, has seen a significant increase in pharmaceutical and biotechnology research and development activities. This growth in drug development has driven the demand for ADME toxicology testing to assess the safety and efficacy of new compounds.
- Growing awareness of toxicology testing
There has been an increasing awareness of the importance of ADME toxicology testing in the drug development process. As regulatory bodies and pharmaceutical companies recognize the significance of early toxicity screening, the demand for ADME testing services has risen.
Opportunities
- Increasing demand for personalized medicine
The trend towards personalized medicine, which involves tailoring medical treatment to the individual characteristics of patients, presents an opportunity for ADME toxicology testing. As drug development becomes more targeted, the need for comprehensive toxicology assessments to ensure safety and efficacy in specific patient populations increases.
- Emergence of advanced technologies
Advances in technologies such as high-throughput screening, computational toxicology, and organ-on-a-chip systems offer opportunities for more efficient and cost-effective ADME testing. Companies in the Asia-Pacific region can capitalize on these technologies to enhance their testing capabilities and attract clients looking for state-of-the-art toxicology services.
Restraints/Challenges
- Stringent regulatory environment
Adhering to evolving and stringent regulatory requirements for drug approval and safety testing can be challenging. Meeting the diverse regulatory standards across different countries within the Asia-Pacific region poses complexities for companies operating in this market.
- Infrastructure and technology gaps
Some countries in the Asia-Pacific region may face challenges related to inadequate infrastructure and technology gaps. Access to state-of-the-art equipment, skilled personnel, and advanced technologies can be limited in certain areas, affecting the quality and efficiency of ADME testing services.
This ADME toxicology testing market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the ADME toxicology testing market contact Data Bridge Market Research for an analyst brief, our team will help you make an informed market decision to achieve market growth.
Recent Developments
- In May 2021, Merck KGaA, a leading science and technology company, announced the extension of its ongoing collaboration with BioMed X, Heidelberg, Germany. As a result of this successful collaboration, multiple innovative discovery projects in new research areas have been started at Merck.
- In April 2021, Agilent Technologies Inc. announced that it has acquired Resolution Bioscience, which is a leader in the development and commercialization of Next Generation Sequencing based precision oncology solutions.
Asia-Pacific ADME Toxicology Testing Market Scope
The ADME toxicology testing market is segmented on the basis of technology, product type, test, method, application, end user, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Technology
- Cell Culture
- High Throughput
- Molecular Imaging
- Omics Technology
Product Type
- Instruments
- Assays and Reagents
- Accessories
- Software Solutions
Test
- In-Vivo
- In-Vitro
Method
- Cellular Assay
- Biochemical Assay
- In-Silica
- Ex-Vivo
Application
- Systemic Toxicity
- Renal Toxicity
- Hepatotoxicity
- Neurotoxicity
- Others
End User
- Biopharmaceutical Companies
- Contract Research Organizations
- Academic and Research Institute
- Others
Distribution Channel
- Direct Tender
- Others
Asia-Pacific ADME Toxicology Testing Market Regional Analysis/Insights
The ADME toxicology testing market is analyzed and market size insights and trends are provided by country, technology, product type, test, method, application, end user, and distribution channel as referenced above.
The countries covered in the market report are Japan, China, India, Australia, South Korea, Singapore, Malaysia, Thailand, Indonesia, Philippines, Vietnam, and rest of Asia-Pacific.
Japan is expected to dominate the market due to rising players in the market and availability of advanced products. India is expected to show fastest growth due to rise in the need of novel drug molecules for toxicology testing.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends, and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Asia-Pacific brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of domestic tariffs, and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The ADME toxicology testing market also provides you with a detailed market analysis for every country's growth in healthcare expenditure for capital equipment, installed base of different kinds of products for the ADME toxicology testing market, the impact of technology using lifeline curves and changes in healthcare regulatory scenarios and their impact on the ADME toxicology testing market.
Competitive Landscape and ADME Toxicology Testing Market Share Analysis
The ADME toxicology testing market competitive landscape provides details by competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width, and breadth, application dominance. The above data points provided are only related to the companies' focus related to the ADME toxicology testing market.
Some of the major players operating in the ADME toxicology testing market are:
- Promega Corporation (U.S.)
- Lonza (Switzerland)
- AAT Bioquest, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Corning Incorporated (U.S.)
- Charles River Laboratories (U.S.)
- LABCYTE INC. (U.S.)
- BioIVT (U.S.)
- Takara Bio Inc. (Japan)
- Agilent Technologies, Inc. (U.S.)
- PromoCell GmbH (Germany)
- Merck KGaA (Germany)
- Bio-Rad Laboratories, Inc. (U.S.)
SKU-