Mercado de fornecimentos para ensaios clínicos no Médio Oriente e África, por serviços (armazenamento, fabrico, embalagem e rotulagem), fase clínica (fase III, fase II, fase IV, fase I), utilizações terapêuticas (oncologia, doenças cardiovasculares, dermatologia, distúrbios metabólicos, Doenças infeciosas , doenças respiratórias, perturbações do SNC e mentais, perturbações do sangue, outras), utilizador final (organizações de investigação por contrato, empresas farmacêuticas e de biotecnologia), tendências e previsões do setor até 2029.
Análise de mercado e insights : Mercado de fornecimentos para ensaios clínicos no Médio Oriente e África
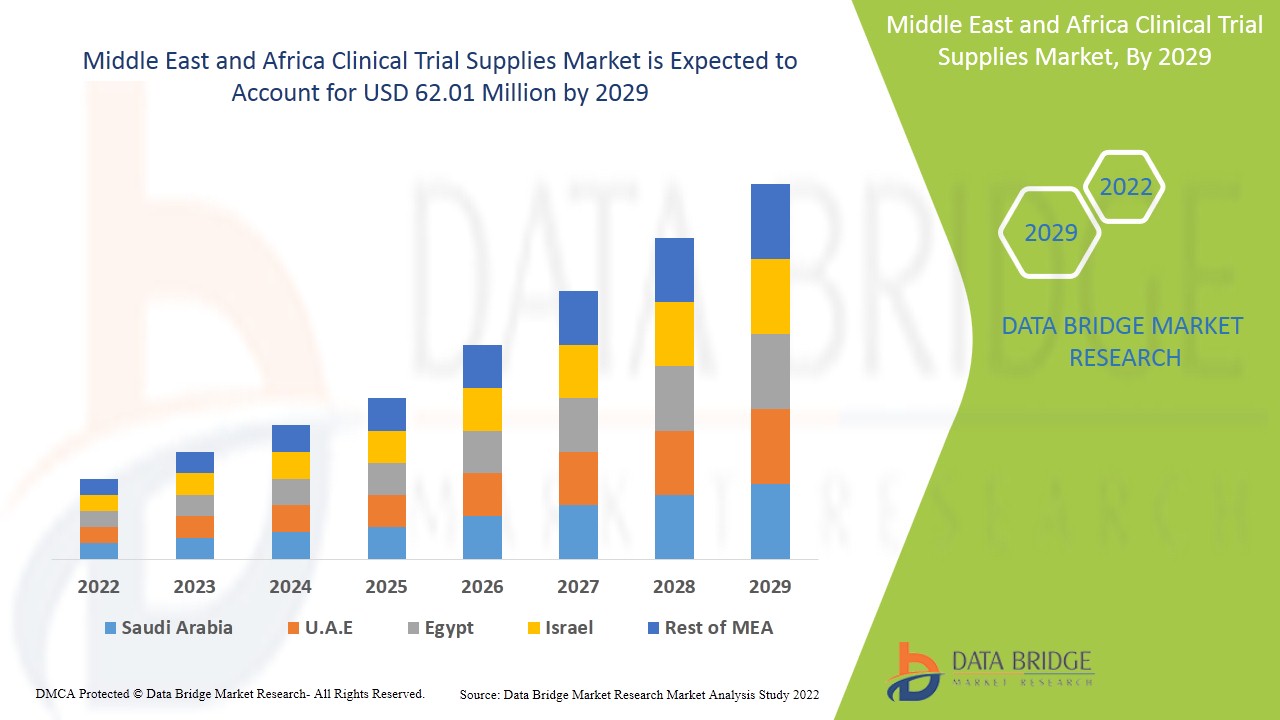
Espera-se que o mercado de fornecimento de ensaios clínicos do Médio Oriente e África ganhe crescimento de mercado no período previsto de 2022 a 2029. A Data Bridge Market Research analisa que o mercado está a crescer com um CAGR de 7,0% no período previsto de 2022 a 2029 e prevê-se que atinja os 62,01 milhões de dólares até 2029. O principal fator que impulsiona o crescimento do mercado de fornecimento de ensaios clínicos é o aumento da procura de ensaios clínicos em todo o mundo, o aumento da incidência de doenças, os fundos governamentais para investimentos em I&D e o desenvolvimento de novos tratamentos, como a medicina personalizada, liderando o mercado de fornecimento para ensaios clínicos irá crescer no futuro.
O ensaio clínico é um estudo de investigação que determina se uma estratégia, tratamento ou dispositivo médico é seguro, eficaz e útil para uso humano. Estes estudos ajudam a descobrir quais as abordagens médicas mais indicadas para determinadas doenças. Um ensaio clínico fornece os melhores dados para fins de tomada de decisão em saúde.
O objetivo do ensaio clínico é estudar padrões científicos rigorosos. Estes padrões protegem os doentes e ajudam a produzir resultados de estudos fiáveis.
Os ensaios clínicos são a última etapa do desenvolvimento de medicamentos num longo e cuidadoso processo de investigação realizado por cientistas ou investigadores para uma doença específica, seja um medicamento ou um dispositivo médico. O processo de desenvolvimento de medicamentos começa geralmente num laboratório, onde os cientistas desenvolvem e testam primeiro novas ideias relacionadas com o tratamento de doenças.
O relatório de mercado de fornecimento de ensaios clínicos no Médio Oriente e em África fornece detalhes sobre a quota de mercado, novos desenvolvimentos e análise de pipeline de produtos, impacto dos participantes do mercado doméstico e localizado, analisa as oportunidades em termos de bolsas de receitas emergentes, alterações nas regulamentações de mercado, aprovações de produtos , estratégias decisões, lançamentos de produtos, expansões geográficas e inovações tecnológicas no mercado. Para compreender a análise e o cenário de mercado, contacte-nos para um Briefing de Analista.
|
Métrica de Reporte |
Detalhes |
|
Período de previsão |
2022 a 2029 |
|
Ano base |
2021 |
|
Anos históricos |
2020 (Personalizável para 2019 - 2014) |
|
Unidades quantitativas |
Receita em milhões de dólares americanos, volumes em unidades, preços em dólares americanos |
|
Segmentos abrangidos |
Por serviços (armazenamento, fabrico, embalagem e rotulagem), fase clínica (fase III, fase II, fase IV, fase I), utilizações terapêuticas (oncologia, doenças cardiovasculares, dermatologia, distúrbios metabólicos, doenças infeciosas, doenças respiratórias, SNC e doenças mentais). |
|
Países abrangidos |
África do Sul, Resto do Médio Oriente e África (MEA) como parte do Médio Oriente e África (MEA) |
|
Atores do mercado abrangidos |
Movianto (EUA), Sharp (EUA), Thermo Fisher Scientific Inc. (EUA), Catalent, Inc (EUA), PCI Pharma Services (EUA), Almac Group (Reino Unido), PAREXEL International Corporation (EUA), Bionical Ltd. (Reino Unido), Alium Medical Limited (Reino Unido), Myonex (Reino Unido), Clinigen Group plc (Reino Unido), Ancillare, LP (EUA), SIRO Clinpharm (Índia) CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC. (Reino Unido) e entre outros. |
Dinâmica do mercado de fornecimentos para ensaios clínicos
Motoristas
- Aumento da procura por ensaios clínicos em todo o mundo
A crescente procura de ensaios clínicos foi de 82% só em países em desenvolvimento, como a América do Norte, Global e Ásia. Estes medicamentos estão disponíveis no mercado após os ensaios clínicos, pelo que todas as empresas realizam ensaios clínicos dependendo do tipo de medicamento ou máquina do dispositivo e, portanto, atuam como um importante impulsionador que resultará na expansão da taxa de crescimento do mercado de tratamento. .
- Aumento da incidência de doenças crónicas
A elevada prevalência de doenças crónicas devido ao rápido crescimento populacional e às infecções entre as pessoas pode ser observada a nível global. Estas doenças desempenham um papel importante no campo dos ensaios clínicos para o desenvolvimento de medicamentos. O medicamento precisa de passar por todas as fases clínicas padrão para estar disponível antes do consumo humano. Portanto, para tratar estas doenças crónicas em humanos, o medicamento tem de ser seguro.
- Fundos governamentais em investimentos em I&D
Os instrumentos, a força de trabalho, a gestão médica em caso de danos para os investigadores, o seguro, o transporte, a taxa do comité de ética, o processamento de dados e outros consumíveis envolvem grandes custos nos ensaios clínicos. Os ensaios clínicos são a avaliação de ideias de prevenção e tratamento de doenças que irão aumentar ainda mais o crescimento do mercado de tratamento.
Oportunidades
- Aumento de ensaios de desenvolvimento de novos medicamentos em países emergentes
O ensaio clínico para a eficácia dos medicamentos é a chave primária para o desenvolvimento de medicamentos para o tratamento de doenças antes do lançamento no mercado para consumo humano. Além disso, os novos medicamentos precisam de cumprir extensões de licença e normas internacionais antes de serem vendidos e distribuídos. O aumento da prevalência e incidência de doenças e o aumento do número de doentes são os fatores que levaram ao surgimento de tendências dos ensaios clínicos para o desenvolvimento de medicamentos nos países em desenvolvimento no último período.
Além disso, os governos dos mercados emergentes (China, Brasil, Rússia, Índia e África do Sul) reformam a saúde pública e garantem um acesso mais acessível aos medicamentos. Estes dois factores a trabalhar em conjunto significam uma maior liberdade para os desenvolvimentos do mercado e uma maior inovação na investigação clínica nos mercados emergentes.
Restrições/Desafios
As reações adversas a medicamentos são os efeitos indesejados ou prejudiciais que podem ser experimentados após a administração de um medicamento em condições normais de utilização em humanos. As reações medicamentosas ocorrem geralmente em icterícia, anemia, erupções cutâneas e levam a uma diminuição da contagem de glóbulos brancos, danos renais e lesões nervosas que causam problemas de visão ou audição.
Muitos dos efeitos adversos podem ser verificados através de exames físicos durante a fase clínica dos testes. Portanto, a comunicação de efeitos adversos durante os ensaios clínicos é o principal fator de restrição para o mercado de inputs. Apesar dos elevados investimentos de tempo e custo no desenvolvimento de produtos biológicos e de novos medicamentos, estima-se que o menor tempo de procedimento e a menor taxa de aprovação de medicamentos estejam a criar um grande desafio para o mercado, o que poderá dificultar o seu crescimento.
Este relatório de mercado de fornecimento de ensaios clínicos fornece detalhes de novos desenvolvimentos recentes, regulamentos comerciais, análise de importação e exportação, análise de produção, otimização da cadeia de valor, quota de mercado, impacto dos participantes do mercado nacional e localizado, analisa as oportunidades em termos de bolsas de receitas emergentes, alterações nas regulamentações do mercado, análise estratégica do crescimento do mercado, tamanho do mercado, crescimento do mercado das categorias, nichos de aplicação e dominância, aprovações de produtos, lançamentos de produtos, expansões geográficas, inovações tecnológicas no mercado. Para mais informações sobre o mercado de fornecimento de ensaios clínicos, contacte a Data Bridge Market Research para um briefing de analista .
Desenvolvimento recente
- Em fevereiro de 2022, a Thermo Fisher Scientific anunciou uma parceria com a Medidata para otimizar a seleção de locais de investigação clínica e acelerar o recrutamento de doentes em ensaios clínicos. Isto melhora o planeamento e a execução de ensaios clínicos para acelerar os ensaios clínicos em que foram gerados conjuntos de dados a partir de 26.000 ensaios clínicos e quase 8 milhões de doentes em mais de 140 países em todo o mundo.
Âmbito do mercado de fornecimentos para ensaios clínicos no Médio Oriente e África
O mercado de fornecimentos para ensaios clínicos do Médio Oriente e África está categorizado com base nos serviços, fase clínica, utilizações terapêuticas e utilizador final. O crescimento entre estes segmentos irá ajudá-lo a analisar segmentos de baixo crescimento nos setores e fornecerá aos utilizadores uma visão geral valiosa do mercado e informações de mercado para tomar decisões estratégicas para identificar as principais aplicações de mercado.
Serviço
- Fabricação
- Distribuição
- Armazenamento
- Embalagem e rotulagem
Com base nos serviços, o mercado de fornecimentos para ensaios clínicos do Médio Oriente e África está segmentado em fabrico, distribuição, armazenamento, embalagem e rotulagem.
Fase clínica
- Fase I
- Fase II
- Fase III
- Fase IV
Com base na fase clínica, o mercado de fornecimentos para ensaios clínicos do Médio Oriente e África está segmentado em fase I, fase II, fase III e fase IV.
Usos terapêuticos
- Oncologia
- SNC
- Transtornos Mentais
- Doenças cardiovasculares
- Doenças Infecciosas
- Doenças Respiratórias
- Distúrbio do sangue
- Dermatologia
- Outros
Com base nas utilizações terapêuticas, o mercado de fornecimentos para ensaios clínicos do Médio Oriente e África está segmentado em oncologia, perturbações do SNC e mentais, doenças cardiovasculares, doenças infeciosas, doenças respiratórias, perturbações metabólicas, perturbações do sangue, dermatologia e outras.
Utilizador final
- Organizações de investigação contratadas
- Empresas farmacêuticas e de biotecnologia
Com base no utilizador final, o mercado de fornecimento de ensaios clínicos do Médio Oriente e de África está segmentado em organizações de investigação por contrato e empresas farmacêuticas e de biotecnologia.
Análise/Insights regionais do mercado de fornecimentos para ensaios clínicos
O mercado de fornecimento para ensaios clínicos do Médio Oriente e África está segmentado em grandes países, como a África do Sul e o restante Médio Oriente e África.
A África do Sul domina o mercado de fornecimento de ensaios clínicos em termos de quota de mercado e receitas de mercado e continuará a aumentar o seu domínio durante o período previsto de 2022-2029. Isto deve-se ao crescente investimento no sector da saúde e ao crescente apoio governamental à infra-estrutura de saúde bem desenvolvida para os seus cidadãos nesta região.
A secção de países do relatório também fornece fatores individuais que impactam o mercado e alterações nas regulamentações do mercado que impactam as tendências atuais e futuras do mercado. Os pontos de dados, como as vendas de produtos novos e de substituição, a demografia dos países e as tarifas de importação e exportação, são alguns dos principais indicadores utilizados para prever o cenário de mercado para países individuais. Além disso, a presença e a disponibilidade de marcas do Médio Oriente e de África e os seus desafios enfrentados devido à elevada concorrência de marcas locais e nacionais, e o impacto dos canais de vendas são considerados ao fornecer uma análise de previsão dos dados do país.
Análise do panorama competitivo e da quota de mercado de fornecimentos para ensaios clínicos no Médio Oriente e em África
O panorama competitivo do mercado de fornecimento de ensaios clínicos no Médio Oriente e em África fornece detalhes por concorrente. Os detalhes incluídos são a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em investigação e desenvolvimento, novas iniciativas de mercado, localizações e instalações de produção, pontos fortes e fracos da empresa, lançamento de produtos, pipelines de testes de produto, aprovações de produto, patentes, largura e amplitude do produto, domínio da aplicação, curva de vida da tecnologia. Os pontos de dados fornecidos acima estão apenas relacionados com o foco das empresas no mercado de fornecimento de ensaios clínicos.
Os principais participantes proeminentes que operam no mercado de fornecimento de ensaios clínicos no Médio Oriente e em África são a Movianto (EUA), a Sharp (EUA), a Thermo Fisher Scientific Inc. (EUA), a Catalent, Inc (EUA), a PCI Pharma Services ( EUA), Almac Group (Reino Unido), PAREXEL International Corporation (EUA), Bionical Ltd. (Reino Unido), Alium Medical Limited (Reino Unido), MYODERM (Reino Unido), Clinigen Group plc (Reino Unido), Ancillare, LP (EUA), SIRO Clinpharm (Índia ) CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC. (EUA) Biocair (Reino Unido) e entre outros.
Metodologia de Investigação : Mercado de Material para Ensaios Clínicos no Médio Oriente e África
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com grandes tamanhos de amostra. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados, que envolve a mineração de dados, análise do impacto das variáveis de dados no mercado e validação primária (especialista do setor). Além disso, os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha do tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise da quota de mercado da empresa, padrões de medição, análise global versus regional e de participação dos fornecedores. Solicite a chamada de um analista em caso de dúvidas adicionais.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTERS FIVE FORCES
5 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: REGULATORY SCENARIO
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING DEMAND FOR CLINICAL TRIALS WORLDWIDE
6.1.2 INCREASING INCIDENCE OF CHRONIC DISEASES
6.1.3 GOVERNMENT FUNDS IN R&D INVESTMENTS
6.1.4 ADVANCEMENT OF TECHNOLOGY IN CLINICAL TRIALS SUPPLIES
6.2 RESTRAINTS
6.2.1 ADVERSE EFFECTS OF CLINICAL TRIALS
6.2.2 TRANSPORTATION ISSUE IN CLINICAL TRIAL SUPPLIES
6.2.3 HIGH COST ASSOCIATED WITH THE CLINICAL TRIALS
6.3 OPPORTUNITIES
6.3.1 INCREASING NEW DRUG DEVELOPMENT TRIALS IN EMERGING COUNTRIES
6.3.2 INCREASING DEMAND FOR INNOVATIVE SOLUTIONS IN CLINICAL TRIALS SERVICES
6.3.3 EVOLUTION IN SUPPLY CHAIN MANAGEMENT FOR CLINICAL TRIALS
6.4 CHALLENGES
6.4.1 LOWER PROCEDURE TIME OF CLINICAL TRIALS APPROVAL
6.4.2 LACK OF SKILLED PERSON TO OPERATE DEVICES DURING CLINICAL TRIALS
7 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES
7.1 OVERVIEW
7.2 STORAGE
7.3 MANUFACTURING
7.4 PACKAGING AND LABELLING
7.5 DISTRIBUTION
8 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASES
8.1 OVERVIEW
8.2 PHASE III
8.3 PHASE II
8.4 PHASE IV
8.5 PHASE I
9 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE
9.1 OVERVIEW
9.2 ONCOLOGY
9.3 CARDIOVASCULAR DISEASES
9.4 DERMATOLOGY
9.5 METABOLIC DISORDERS
9.6 INFECTIOUS DISEASES
9.7 RESPIRATORY DISEASES
9.8 CNS AND MENTAL DISORDERS
9.9 BLOOD DISORDERS
9.1 OTHERS
10 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY END USER
10.1 OVERVIEW
10.2 CONTRACT RESEARCH ORGANIZATIONS
10.3 PHARMACEUTICAL AND BIOTECHNOLOGY COMPANIES
11 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY REGION
11.1 MIDDLE EAST & AFRICA
11.1.1 SOUTH AFRICA
11.1.2 REST OF MIDDLE EAST & AFRICA
12 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 THERMO FISHER SCIENTIFIC INC.
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENT
14.1.5.1 PARTNERSHIP
14.2 ALMAC GROUP
14.2.1 COMPANY SNAPSHOT
14.2.2 COMPANY SHARE ANALYSIS
14.2.3 PRODUCT PORTFOLIO
14.2.4 RECENT DEVELOPMENTS
14.3 CATALENT INC.
14.3.1 COMPANY SNAPSHOT
14.3.2 REVENUE ANALYSIS
14.3.3 COMPANY SHARE ANALYSIS
14.3.4 SERVICE PORTFOLIO
14.3.5 RECENT DEVELOPMENT
14.3.5.1 SERVICE EXPANSION
14.4 CLINIGEN GROUP PLC
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENT
14.4.5.1 PARTNERSHIP
14.5 MOVIANTO
14.5.1 COMPANY SNAPSHOT
14.5.2 COMPANY SHARE ANALYSIS
14.5.3 SERVICE PORTFOLIO
14.5.4 RECENT DEVELOPMENT
14.5.4.1 ACQUISITION
14.6 PCI PHARMA SERVICES
14.6.1 COMPANY SNAPSHOT
14.6.2 SERVICE PORTFOLIO
14.6.3 RECENT DEVELOPMENTS
14.7 SHARP
14.7.1 COMPANY SNAPSHOT
14.7.2 SERVICE PORTFOLIO
14.7.3 RECENT DEVELOPMENT
14.8 ALIUM MEDICAL LIMITED
14.8.1 COMPANY SNAPSHOT
14.8.2 SERVICE PORTFOLIO
14.8.3 RECENT DEVELOPMENT
14.9 ANCILLARE, LP
14.9.1 COMPANY SNAPSHOT
14.9.2 SERVICE PORTFOLIO
14.9.3 RECENT DEVELOPMENT
14.1 BIOCAIR
14.10.1 COMPANY SNAPSHOT
14.10.2 SERVICE PORTFOLIO
14.10.3 RECENT DEVELOPMENTS
14.11 BIONICAL LTD.
14.11.1 COMPANY SNAPSHOT
14.11.2 SERVICE PORTFOLIO
14.11.3 RECENT DEVELOPMENT
14.11.3.1 SERVICE LAUNCH
14.12 CLINICAL SUPPLIES MANAGEMENT HOLDINGS,INC
14.12.1 COMPANY SNAPSHOT
14.12.2 SERVICE PORTFOLIO
14.12.3 RECENT DEVELOPMENT
14.13 KLIFO
14.13.1 COMPANY SNAPSHOT
14.13.2 SERVICE PORTFOLIO
14.13.3 RECENT DEVELOPMENTS
14.13.3.1 ACQUISTION
14.14 MYONEX
14.14.1 COMPANY SNAPSHOT
14.14.2 SERVICE PORTFOLIO
14.14.3 RECENT DEVELOPMENT
14.15 PAREXEL INTERNATIONAL CORPORATION
14.15.1 COMPANY SNAPSHOT
14.15.2 SERVICE PORTFOLIO
14.15.3 RECENT DEVELOPMENT
14.15.3.1 COLLABORATION
14.16 SIRO CLINPHARM PRIVATE LIMITED
14.16.1 COMPANY SNAPSHOT
14.16.2 SERVICE PORTFOLIO
14.16.3 RECENT DEVELOPMENTS
15 QUESTIONNAIRE
16 RELATED REPORTS
Lista de Tabela
TABLE 1 LOCATIONS OF REGISTERED STUDIES
TABLE 2 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA STORAGE IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA MANUFACTURING IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA PACKAGING AND LABELLING IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA DISTRIBUTION IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA PHASE III IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA PHASE II IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA PHASE IV IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA PHASE I IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA ONCOLOGY IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA CARDIOVASCULAR DISEASES IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA DERMATOLOGY IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA METABOLIC DISORDERS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA INFECTIOUS DISEASES IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA RESPIRATORY DISEASES IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA CNS AND MENTAL DISORDERS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA BLOOD DISORDERS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA OTHERS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA CONTRACT RESEARCH ORGANIZATIONS IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA PHARMACEUTICAL AND BIOTECHNOLOGY COMPANIES IN CLINICAL TRIAL SUPPLIES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 30 SOUTH AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 31 SOUTH AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY CLINICAL PHASE, 2020-2029 (USD MILLION)
TABLE 32 SOUTH AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY THERAPEUTIC USE, 2020-2029 (USD MILLION)
TABLE 33 SOUTH AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 34 REST OF MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
Lista de Figura
FIGURE 1 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPLLIES MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPLLIES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: DBMR VENDOR SHARE ANALYSIS
FIGURE 9 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: SEGMENTATION
FIGURE 10 NORTH AMERICA IS EXPECTED TO DOMINATE THE MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 11 RISING DEMAND FOR CLINICAL TRIALS WORLDWIDE AND INCREASING INCIDENCES OF DISEASES IS DRIVING THE MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 STORAGE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET
FIGURE 14 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES, 2021
FIGURE 15 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES, 2022-2029 (USD MILLION)
FIGURE 16 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES, CAGR (2022-2029)
FIGURE 17 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES, LIFELINE CURVE
FIGURE 18 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY CLINICAL PHASE, 2021
FIGURE 19 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY CLINICAL PHASE, 2022-2029 (USD MILLION)
FIGURE 20 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY CLINICAL PHASE, CAGR (2022-2029)
FIGURE 21 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY CLINICAL PHASE, LIFELINE CURVE
FIGURE 22 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY THERAPEUTIC USE, 2021
FIGURE 23 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY THERAPEUTIC USE, 2022-2029 (USD MILLION)
FIGURE 24 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY THERAPEUTIC USE, CAGR (2022-2029)
FIGURE 25 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY THERAPEUTIC USE, LIFELINE CURVE
FIGURE 26 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY END USER, 2021
FIGURE 27 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 28 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY END USER, CAGR (2022-2029)
FIGURE 29 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: SNAPSHOT (2021)
FIGURE 31 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY COUNTRY (2021)
FIGURE 32 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 33 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 34 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: BY SERVICES (2022-2029)
FIGURE 35 MIDDLE EAST & AFRICA CLINICAL TRIAL SUPPLIES MARKET: COMPANY SHARE 2021 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.