Global Molecular Point Of Care Testing Using Naat Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
37.93 Billion
USD
86.17 Billion
2024
2032
USD
37.93 Billion
USD
86.17 Billion
2024
2032
| 2025 –2032 | |
| USD 37.93 Billion | |
| USD 86.17 Billion | |
|
|
|
|
Segmentação do mercado global de testes moleculares no local de atendimento (usando NAAT), porProduto (instrumentos, consumíveis e reagentes), Indicação (testes para infecções respiratórias, infecções sexualmente transmissíveis (ISTs), infecções do trato gastrointestinal e outros), Usuário final (laboratórios, hospitais, clínicas, centros ambulatoriais, assistência domiciliar, residências assistidas e outros), Modalidade de teste (testes com prescrição médica e testes sem prescrição), Canal de distribuição (farmácia hospitalar, farmácia de varejo e farmácia online) - Tendências e previsões do setor até 2032
Tamanho do mercado de testes moleculares no local de atendimento (usando NAAT)
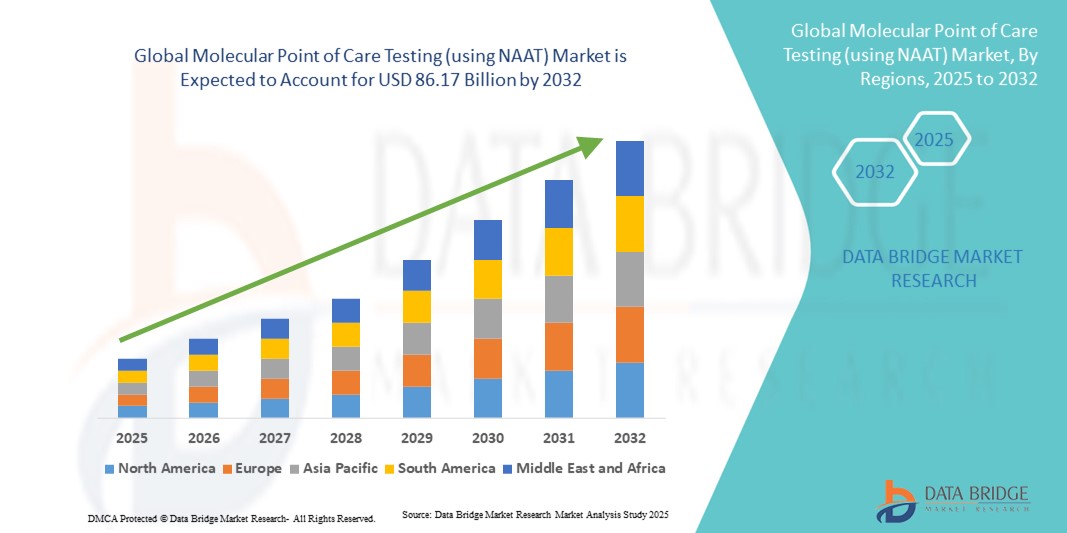
- O mercado global de testes moleculares no local de atendimento (utilizando NAAT) foi avaliado em US$ 37,93 bilhões em 2024 e deverá atingir US$ 86,17 bilhões até 2032 , com uma taxa de crescimento anual composta (CAGR) de 1,08% durante o período de previsão.
- Esse crescimento de mercado é impulsionado principalmente pela crescente prevalência de doenças infecciosas , pela demanda por testes diagnósticos rápidos e precisos e pelos avanços nas tecnologias de diagnóstico molecular.
- Além disso, a mudança para um sistema de saúde descentralizado e a crescente adoção de testes multiplex estão contribuindo para a expansão do mercado de testes moleculares no ponto de atendimento (POCT). Esses fatores, em conjunto, posicionam os testes moleculares no ponto de atendimento como um componente fundamental no diagnóstico moderno, oferecendo detecção oportuna e precisa de diversas condições de saúde.
Análise de mercado de testes moleculares no local de atendimento (usando NAAT)
- Os testes moleculares no local de atendimento (POCT, na sigla em inglês), que utilizam a técnica de amplificação de ácidos nucleicos (NAAT, na sigla em inglês), proporcionam a detecção rápida e precisa de doenças infecciosas e outras condições médicas no próprio local do paciente ou próximo a ele, tornando-se um componente crucial do diagnóstico moderno em hospitais, clínicas e serviços de saúde descentralizados.
- A crescente adoção de testes moleculares no local de atendimento (POCT) é impulsionada principalmente pela crescente prevalência de doenças infecciosas, pela demanda cada vez maior por diagnósticos rápidos e precisos e pelos avanços tecnológicos em dispositivos portáteis e fáceis de usar para testes moleculares.
- A América do Norte dominou o mercado de testes moleculares no ponto de atendimento (POCT) com a maior participação de receita, de 39% em 2024, impulsionada pela adoção precoce de tecnologias de diagnóstico avançadas, altos gastos com saúde e forte presença de importantes players do setor. Os EUA apresentaram crescimento substancial devido à integração de testes baseados em NAAT em hospitais, clínicas e serviços de emergência.
- A região Ásia-Pacífico deverá ser a de crescimento mais rápido durante o período de previsão, impulsionada pelo aumento dos investimentos em infraestrutura de saúde, pela crescente conscientização sobre soluções de diagnóstico rápido e pela demanda cada vez maior por testes no local de atendimento em economias emergentes.
- O segmento de testes para infecções respiratórias dominou o mercado de testes moleculares no ponto de atendimento (POCT) com uma participação de 43,2% em 2024, devido à alta prevalência de doenças respiratórias infecciosas e à necessidade clínica de diagnósticos rápidos e precisos para orientar o tratamento oportuno e as medidas de contenção.
Escopo do relatório e segmentação do mercado de testes moleculares no local de atendimento (usando NAAT)
|
Atributos |
Testes moleculares no local de atendimento (usando NAAT): Principais insights de mercado |
|
Segmentos abrangidos |
|
|
Países abrangidos |
América do Norte
Europa
Ásia-Pacífico
Oriente Médio e África
Ámérica do Sul
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além das informações sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado elaborados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, análises de preços, análises de participação de marcas, pesquisas com consumidores, análises demográficas, análises da cadeia de suprimentos, análises da cadeia de valor, visão geral de matérias-primas/insumos, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de testes moleculares no local de atendimento (usando NAAT)
Avanços em testes rápidos, multiplexados e habilitados por IA
- Uma tendência significativa e crescente no mercado global de testes moleculares no ponto de atendimento (POCT) é o desenvolvimento de dispositivos NAAT multiplexados e rápidos, além de plataformas de diagnóstico com inteligência artificial (IA). Essas plataformas aprimoram a velocidade, a precisão e a usabilidade dos testes no ponto de atendimento em hospitais, clínicas e ambientes de saúde descentralizados.
- Por exemplo, o teste Xpert Xpress SARS-CoV-2/Gripe/VSR integra a detecção de múltiplos patógenos em uma única execução, reduzindo o tempo de resposta e otimizando o fluxo de trabalho nos locais de atendimento ao paciente.
- A integração da IA em testes moleculares no ponto de atendimento (POCT) possibilita recursos como a interpretação automatizada dos resultados dos testes, análises preditivas para surtos de doenças e controle de qualidade inteligente, melhorando a confiabilidade do diagnóstico e reduzindo erros humanos.
- Essas plataformas baseadas em IA permitem que os profissionais de saúde gerenciem múltiplos parâmetros de diagnóstico simultaneamente, fornecendo resultados mais rápidos para condições críticas e reduzindo a dependência de laboratórios centralizados.
- Essa tendência em direção a soluções de teste mais rápidas, precisas e interconectadas está remodelando as expectativas para diagnósticos no ponto de atendimento. Consequentemente, empresas como Abbott e Roche estão desenvolvendo dispositivos NAAT com inteligência artificial, recursos multiplex e interfaces amigáveis para a equipe clínica.
- A demanda por dispositivos moleculares de POCT (Point-of-Care Testing) que oferecem detecção multiplex e funcionalidades baseadas em IA está crescendo rapidamente tanto em ambientes hospitalares quanto ambulatoriais, à medida que os profissionais de saúde priorizam cada vez mais diagnósticos rápidos e confiáveis.
Dinâmica do mercado de testes moleculares no local de atendimento (usando NAAT)
Motorista
Aumento da demanda devido à carga de doenças infecciosas e à testagem descentralizada.
- O aumento da prevalência de doenças infecciosas em todo o mundo, aliado à tendência para a descentralização da prestação de cuidados de saúde, é um fator significativo para a crescente adoção de testes moleculares no ponto de atendimento (POCT) utilizando testes de amplificação de ácidos nucleicos (NAAT).
- Por exemplo, em 2024, a Cepheid lançou aplicações expandidas de sua plataforma GeneXpert para incluir infecções sexualmente transmissíveis e painéis respiratórios, refletindo os esforços para atender às crescentes necessidades de diagnóstico no local de atendimento.
- À medida que os profissionais de saúde buscam diagnósticos mais rápidos e tratamentos oportunos, os testes rápidos baseados em NAAT oferecem alta sensibilidade, especificidade e resultados rápidos, representando uma alternativa atraente aos testes laboratoriais centralizados tradicionais.
- Além disso, a crescente ênfase no gerenciamento de surtos e na preparação para emergências está tornando os testes moleculares no local de atendimento um componente essencial dos programas de controle de doenças infecciosas, permitindo decisões rápidas de isolamento e tratamento.
- A conveniência dos testes próximos ao paciente, os requisitos mínimos de processamento de amostras e a capacidade de gerenciar vários testes simultaneamente são fatores-chave que impulsionam a adoção de testes moleculares no local de atendimento baseados em NAAT em hospitais, clínicas e ambientes de campo.
Restrição/Desafio
Obstáculos regulatórios e limitações operacionais
- A conformidade regulatória e os rigorosos processos de aprovação para dispositivos moleculares de POCT representam desafios significativos para a expansão do mercado, uma vez que as plataformas baseadas em NAAT devem atender a padrões rigorosos de precisão, segurança e confiabilidade antes da comercialização.
- Por exemplo, atrasos na aprovação da FDA ou da CE podem retardar o lançamento de produtos inovadores de POCT (Point-of-Care Testing), limitando o acesso a diagnósticos avançados em cenários onde o tempo é crucial.
- Limitações operacionais, como a necessidade de pessoal treinado, manutenção dos equipamentos e fornecimento de consumíveis, podem restringir a adoção em ambientes com recursos limitados, apesar das vantagens tecnológicas dos dispositivos NAAT.
- Além disso, o custo relativamente alto de dispositivos POCT multiplexados avançados ou com inteligência artificial, em comparação com os testes rápidos convencionais, pode ser uma barreira para clínicas pequenas ou regiões de baixa renda, limitando sua ampla implementação.
- Embora os custos estejam diminuindo gradualmente, o preço elevado percebido para plataformas moleculares sofisticadas de testes no local de atendimento pode dificultar a adoção em ambientes de saúde com orçamentos limitados.
- Superar esses desafios por meio de processos regulatórios simplificados, treinamento de pessoal e desenvolvimento de dispositivos com boa relação custo-benefício será vital para o crescimento sustentado do mercado.
Escopo de mercado para testes moleculares no local de atendimento (usando NAAT)
O mercado é segmentado com base no produto, indicação, usuário final, método de teste e canal de distribuição.
- Por produto
Com base no produto, o mercado de testes moleculares no ponto de atendimento (POCT) é segmentado em instrumentos e consumíveis e reagentes. O segmento de instrumentos dominou o mercado com a maior participação na receita, de 55,3% em 2024, impulsionado pelo papel crucial dos dispositivos automatizados de amplificação de ácidos nucleicos (NAAT) na entrega de resultados rápidos e precisos. As instituições de saúde priorizam a adoção de instrumentos devido ao seu alto rendimento, confiabilidade e integração com sistemas de informação laboratorial . Os investimentos em instrumentos modernos permitem que hospitais e laboratórios otimizem os fluxos de trabalho, melhorem a eficiência e reduzam o tempo de resposta para diagnósticos de doenças infecciosas. O segmento se beneficia de inovações tecnológicas, como instrumentos portáteis e compactos adequados para testes descentralizados. Os instrumentos são frequentemente combinados com plataformas de software para facilitar a geração automatizada de relatórios e o controle de qualidade. O suporte técnico contínuo e os serviços de manutenção oferecidos pelos fabricantes também contribuem para a adoção.
O segmento de consumíveis e reagentes deverá apresentar a taxa de crescimento anual composta (CAGR) mais rápida, de 12,5%, entre 2025 e 2032, impulsionado pela necessidade recorrente de cartuchos, kits de teste e reagentes utilizados em testes rápidos baseados em NAAT (amplificação de ácidos nucleicos). Os consumíveis são essenciais para as operações diárias de testes em hospitais, clínicas e laboratórios, gerando receita contínua para os fornecedores. A crescente conscientização sobre testes de doenças infecciosas e o aumento da frequência de testes impulsionam a demanda. Ensaios multiplexados que exigem reagentes especializados aceleram ainda mais o crescimento. Inovações tecnológicas que aprimoram a estabilidade e a facilidade de uso dos reagentes contribuem para a adoção. O segmento também se beneficia de melhorias na cadeia de suprimentos que reduzem os prazos e custos de entrega.
- Por indicação
Com base na indicação, o mercado é segmentado em testes para infecções respiratórias, infecções sexualmente transmissíveis (ISTs), infecções do trato gastrointestinal e outras. O segmento de testes para infecções respiratórias dominou o mercado com uma participação de 43,2% em 2024, devido à alta prevalência de patógenos como SARS-CoV-2, influenza e VSR. A detecção rápida e precisa de infecções respiratórias é crucial em hospitais, clínicas e unidades de emergência para o tratamento e contenção oportunos. Os testes rápidos baseados em NAAT oferecem alta sensibilidade e especificidade, tornando-se o método preferido para infecções respiratórias. A adoção é ainda impulsionada por programas governamentais de monitoramento e controle de surtos. Hospitais e laboratórios integram esses testes aos fluxos de trabalho de emergência para uma tomada de decisão rápida. Inovações contínuas em painéis multiplexados aumentam a produtividade e a eficiência.
O segmento de testes de ISTs deverá apresentar a taxa de crescimento anual composta (CAGR) mais rápida, de 13,1%, entre 2025 e 2032, impulsionado pela crescente conscientização, programas de rastreamento e aumento da incidência de infecções sexualmente transmissíveis. Os testes rápidos baseados em NAAT permitem a detecção rápida, confidencial e precisa de ISTs, sendo ideais para clínicas e testes domiciliares. Os ensaios multiplexados, que detectam múltiplas ISTs em um único teste, aceleram a adoção. A expansão dos autotestes e das iniciativas de saúde sexual impulsiona a demanda. A integração com plataformas de telemedicina aprimora a emissão de resultados e o aconselhamento. O aumento das parcerias entre empresas de diagnóstico e profissionais de saúde impulsiona ainda mais o crescimento do mercado.
- Por usuário final
Com base no usuário final, o mercado de testes moleculares no ponto de atendimento (POCT) é segmentado em laboratórios, hospitais, clínicas, centros ambulatoriais, assistência domiciliar, residências assistenciais e outros. O segmento de hospitais dominou o mercado com a maior participação na receita, de 48,6% em 2024, devido ao alto volume de pacientes e à necessidade de diagnósticos rápidos. Os hospitais investem em dispositivos NAAT avançados para melhorar o fluxo de trabalho, gerenciar surtos infecciosos e apoiar o atendimento de emergência. A integração com os sistemas de informação hospitalar aumenta a eficiência operacional. O segmento se beneficia de inovações tecnológicas contínuas e incentivos governamentais. Os hospitais geralmente necessitam de instrumentos que possam lidar com múltiplos tipos de testes. Contratos de longo prazo com fornecedores garantem o fornecimento consistente de instrumentos e consumíveis.
O segmento de testes domiciliares deverá apresentar a taxa de crescimento anual composta (CAGR) mais rápida, de 14,2%, entre 2025 e 2032, impulsionado pela crescente demanda por soluções de testes em casa, conveniência e privacidade. Os kits de NAAT para uso domiciliar permitem que os pacientes façam o teste sem precisar ir a clínicas ou hospitais. O autoteste para infecções respiratórias e ISTs impulsiona a adoção. A integração com aplicativos para smartphones facilita a interpretação dos resultados e o envio de relatórios de telemedicina. Campanhas de conscientização e parcerias com provedores de telemedicina ampliam o alcance. A crescente aceitação dos testes domiciliares pelas autoridades de saúde sustenta o crescimento contínuo.
- Por método de teste
Com base no método de teste, o mercado é segmentado em testes com prescrição médica e testes de venda livre (OTC). O segmento de testes com prescrição médica dominou o mercado com uma participação de 62,3% em 2024, impulsionado por requisitos regulatórios e supervisão profissional. Os testes com prescrição médica oferecem precisão, confiabilidade e interpretação clínica, especialmente em hospitais e clínicas. A integração com os fluxos de trabalho da área da saúde garante a segurança do paciente e o controle de qualidade. A adoção é expressiva para doenças infecciosas de alto risco, onde a orientação de especialistas é necessária. Os dispositivos são frequentemente conectados às redes hospitalares para rastreamento e emissão de relatórios de resultados. Os fabricantes continuam a aprimorar a usabilidade, atendendo aos padrões regulatórios.
O segmento de testes de venda livre (OTC) deverá apresentar a taxa de crescimento anual composta (CAGR) mais rápida, de 13,5%, entre 2025 e 2032, impulsionado pela crescente demanda por autotestes para infecções respiratórias, ISTs e infecções gastrointestinais. Kits fáceis de usar e a interpretação via smartphone melhoram a acessibilidade. Os consumidores preferem soluções OTC pela conveniência, privacidade e redução de consultas clínicas. Programas de conscientização e a integração com a telemedicina impulsionam a adoção. Varejistas e farmácias online aumentam a disponibilidade. O segmento se beneficia de kits de teste simplificados e pré-embalados, projetados para uso doméstico.
- Por canal de distribuição
Com base no canal de distribuição, o mercado é segmentado em farmácia hospitalar, farmácia de varejo e farmácia online. O segmento de farmácia hospitalar dominou o mercado com uma participação de 57,4% em 2024, impulsionado pela aquisição direta para testes em pacientes internados e ambulatoriais. Os hospitais utilizam os canais de farmácia para o fornecimento consistente de dispositivos NAAT, consumíveis e reagentes. Acordos de compra em grande volume reduzem custos e garantem o reabastecimento oportuno. A integração com os sistemas de cadeia de suprimentos hospitalares minimiza a falta de estoque. Os hospitais frequentemente recebem suporte técnico e treinamento por meio de contratos com farmácias. Parcerias com fabricantes fortalecem a disponibilidade de produtos e serviços.
O segmento de farmácias online deverá apresentar a taxa de crescimento anual composta (CAGR) mais rápida, de 15,1%, entre 2025 e 2032, impulsionado pela facilidade de compra, entrega em domicílio de kits de teste de amplificação de ácidos nucleicos (NAAT) e crescente adoção da telemedicina. As plataformas online permitem a compra discreta de testes, tanto com receita quanto sem receita. Campanhas de marketing digital ampliam o conhecimento do consumidor. A integração com aplicativos de telessaúde permite o envio contínuo de resultados. Os consumidores preferem cada vez mais o acesso online pela conveniência e privacidade. O crescimento do comércio eletrônico e as melhorias logísticas sustentam a rápida expansão desse canal de distribuição.
Análise Regional do Mercado de Testes Moleculares no Local de Atendimento (utilizando NAAT)
- A América do Norte dominou o mercado de testes moleculares no ponto de atendimento (POCT) com a maior participação de receita, de 39% em 2024, impulsionada pela adoção precoce de tecnologias de diagnóstico avançadas, altos gastos com saúde e forte presença de importantes players do setor. Os EUA apresentaram crescimento substancial devido à integração de testes baseados em NAAT em hospitais, clínicas e serviços de emergência.
- Os profissionais de saúde da região priorizam testes rápidos, precisos e confiáveis, tornando os dispositivos de diagnóstico no local de atendimento (POCT) baseados em NAAT essenciais para hospitais, clínicas e serviços de emergência. Os EUA, em particular, estão testemunhando um crescimento substancial devido à integração de dispositivos NAAT em hospitais, clínicas ambulatoriais e testes domiciliares, impulsionado por inovações tanto de empresas de diagnóstico consolidadas quanto de startups.
- A ampla adoção é ainda mais favorecida por uma infraestrutura de saúde robusta, altos gastos com saúde e uma população tecnologicamente avançada.
Análise do mercado de testes moleculares no local de atendimento (usando NAAT) nos EUA
O mercado de testes moleculares POCT nos EUA detinha a maior participação de receita, com 79% em 2024, na América do Norte, impulsionado pela rápida adoção de tecnologias de diagnóstico avançadas e pela crescente necessidade de detecção oportuna de doenças infecciosas. Os profissionais de saúde estão priorizando cada vez mais os testes baseados em NAAT para infecções respiratórias , ISTs e infecções gastrointestinais devido à sua alta precisão e rapidez nos resultados. A crescente tendência de cuidados de saúde descentralizados e testes domiciliares impulsiona ainda mais o mercado. Além disso, a integração de dispositivos de POCT molecular com sistemas de informação hospitalar e plataformas de telemedicina contribui significativamente para a expansão do mercado. A forte presença de importantes empresas de diagnóstico e as contínuas inovações tecnológicas também sustentam o crescimento contínuo.
Análise do mercado europeu de testes moleculares no local de atendimento (utilizando NAAT):
Prevê-se que o mercado europeu de testes moleculares no local de atendimento (POCT) expanda a uma taxa de crescimento anual composta (CAGR) substancial durante o período de previsão, impulsionado principalmente pela crescente demanda por diagnósticos rápidos e pela incidência crescente de doenças infecciosas. Iniciativas governamentais que promovem testes no local de atendimento e a digitalização da saúde estão incentivando a adoção em hospitais, clínicas e laboratórios. O crescimento também é sustentado pela urbanização e pela crescente prevalência de infecções multirresistentes. Os prestadores de serviços de saúde europeus estão adotando cada vez mais testes baseados em NAAT para o gerenciamento oportuno de doenças e o controle de surtos. Tanto instalações de saúde residenciais quanto comerciais estão integrando dispositivos POCT para aprimorar o atendimento ao paciente. O mercado está testemunhando um crescimento significativo em países como Alemanha, França e Itália.
Análise do mercado de testes moleculares no local de atendimento no Reino Unido (usando NAAT):
Prevê-se que o mercado de testes moleculares POCT no Reino Unido cresça a uma taxa composta de crescimento anual (CAGR) notável durante o período de previsão, impulsionado pela crescente demanda por diagnósticos rápidos e soluções de testes descentralizadas. A crescente conscientização entre os profissionais de saúde sobre os benefícios dos testes baseados em NAAT, como alta sensibilidade e especificidade, está incentivando a adoção em hospitais e clínicas. Iniciativas governamentais para a detecção precoce e o controle de doenças infecciosas também contribuem para a expansão do mercado. A sólida infraestrutura de saúde e os robustos sistemas de saúde eletrônica do país aprimoram a integração de dispositivos e a emissão de resultados. A crescente adoção nos setores de saúde público e privado estimula o crescimento do mercado. Espera-se que as iniciativas de telemedicina e testes domiciliares impulsionem ainda mais a adoção no Reino Unido.
Análise do mercado de testes moleculares no local de atendimento (utilizando NAAT) na Alemanha
O mercado alemão de testes moleculares POCT deverá expandir a uma taxa de crescimento anual composta (CAGR) considerável durante o período de previsão, impulsionado pela crescente prevalência de doenças infecciosas e pela demanda por diagnósticos rápidos. A infraestrutura de saúde avançada da Alemanha, a forte ênfase na inovação tecnológica e o alto nível de conscientização entre os profissionais de saúde promovem a adoção de POCT baseados em NAAT. A integração de dispositivos POCT com sistemas de informação hospitalar e redes de laboratórios aumenta a eficiência operacional. Há uma crescente preferência por testes descentralizados em clínicas ambulatoriais e centros de atendimento ambulatorial. A adoção tanto em serviços de saúde residenciais quanto em instalações de diagnóstico comerciais está aumentando. A pesquisa e o desenvolvimento contínuos, bem como a fabricação local de instrumentos e consumíveis para POCT, fortalecem ainda mais o crescimento do mercado.
Análise do mercado de testes moleculares no local de atendimento (utilizando NAAT) na região Ásia-Pacífico
O mercado de testes moleculares POCT na região Ásia-Pacífico está preparado para crescer à taxa composta de crescimento anual (CAGR) mais rápida, de 23,5%, durante o período de previsão de 2025 a 2032, impulsionado pela crescente incidência de doenças infecciosas, pelo aumento dos investimentos em saúde e pela expansão da infraestrutura de testes em países como China, Japão e Índia. O foco crescente da região em soluções de testes descentralizadas e domiciliares está impulsionando a adoção. Programas governamentais que promovem a saúde digital e o monitoramento de doenças infecciosas apoiam a expansão do mercado. A presença de fabricantes locais garante dispositivos e consumíveis NAAT a preços acessíveis. A crescente urbanização, a adoção de tecnologia e a maior conscientização sobre o diagnóstico precoce impulsionam ainda mais o crescimento. A demanda em hospitais, clínicas e serviços de assistência domiciliar está aumentando de forma constante.
Análise do mercado japonês de testes moleculares no local de atendimento (utilizando NAAT):
O mercado japonês de testes moleculares POCT está ganhando impulso devido aos altos padrões de saúde, à adoção de tecnologias de diagnóstico avançadas e à crescente conscientização sobre testes rápidos. O país prioriza a detecção precisa e oportuna de doenças infecciosas, impulsionando a adoção em hospitais, clínicas e ambientes de atendimento domiciliar. A integração de dispositivos NAAT com redes hospitalares e plataformas de telemedicina aumenta a eficiência. O envelhecimento da população aumenta ainda mais a demanda por soluções de teste fáceis de usar e acessíveis. Inovações contínuas em dispositivos NAAT multiplexados e portáteis sustentam o mercado. A crescente preferência por cuidados de saúde preventivos e diagnósticos digitais acelera a adoção tanto no setor de saúde residencial quanto no comercial.
Análise do mercado de testes moleculares no local de atendimento (usando NAAT) na Índia
O mercado de testes moleculares POCT na Índia representou a maior fatia de receita na região Ásia-Pacífico em 2024, devido à alta prevalência de doenças infecciosas no país, à expansão da classe média e ao aumento da conscientização sobre saúde. A rápida urbanização e as iniciativas governamentais voltadas para a saúde inteligente e o diagnóstico digital são os principais impulsionadores desse crescimento. Hospitais, clínicas e serviços de atendimento domiciliar estão adotando cada vez mais dispositivos POCT baseados em NAAT para diagnósticos e tratamentos mais rápidos. Dispositivos acessíveis de fabricantes locais aumentam a disponibilidade em áreas urbanas e semiurbanas. A crescente demanda por soluções de testes multiplexados acelera ainda mais a adoção desses dispositivos. Programas robustos de saúde pública e parcerias com empresas de diagnóstico continuam impulsionando o crescimento do mercado.
Participação de mercado dos testes moleculares no local de atendimento (usando NAAT)
O setor de testes moleculares no local de atendimento (utilizando NAAT) é liderado principalmente por empresas consolidadas, incluindo:
- Thermo Fisher Scientific Inc. (EUA)
- Hologic, Inc. (EUA)
- BD (EUA)
- F. Hoffmann-La Roche Ltda. (Suíça)
- Abbott (EUA)
- QIAGEN (Países Baixos)
- BIOMÉRIEUX (França)
- Danaher (EUA)
- Illumina, Inc. (EUA)
- Corporação Sysmex (Japão)
- Siemens Healthineers AG (Alemanha)
- Seegene Inc. (Coreia do Sul)
- Guardant Health, Inc. (EUA)
- Labcorp (EUA)
- Exact Sciences Corporation (EUA)
- 10x Genomics, Inc. (EUA)
- DNA Genotek Inc. (Canadá)
- PathoNostics (Países Baixos)
- Molbio Diagnostics Limited. (Índia)
Quais são os desenvolvimentos recentes no mercado global de testes moleculares no local de atendimento (usando NAAT)?
- Em julho de 2025, a BD (Becton, Dickinson and Company) recebeu a aprovação 510(k) da FDA para seu sistema BD Veritor™ para SARS-CoV-2, um teste digital projetado para detectar antígenos da COVID-19 em indivíduos sintomáticos em cerca de 15 minutos em locais de atendimento, como consultórios médicos, centros de atendimento de urgência e clínicas de varejo.
- Em outubro de 2024, a Organização Mundial da Saúde (OMS) aprovou o primeiro teste diagnóstico para varíola dos macacos (mpox, anteriormente conhecida como varíola símia) para uso emergencial. Essa aprovação visa ampliar o acesso global a diagnósticos rápidos e precisos para mpox, principalmente em locais com recursos limitados. O teste utiliza tecnologia de amplificação de ácido nucleico, permitindo testes no local de atendimento com alta sensibilidade e especificidade.
- Em abril de 2024, a Roche Diagnostics lançou o sistema cobas® 5800, uma plataforma de automação de testes moleculares de última geração, projetada para melhorar a produtividade e reduzir erros em laboratórios. O sistema oferece ensaios padronizados e soluções escaláveis, tornando-o adequado para diversos volumes e combinações de testes. Ao aprimorar a automação em diagnósticos moleculares, o sistema cobas® 5800 visa otimizar os fluxos de trabalho e garantir resultados consistentes em diferentes ambientes de teste.
- Em março de 2023, a QuidelOrtho Corporation anunciou que obteve uma autorização De Novo da Food and Drug Administration (FDA) dos EUA, permitindo à empresa comercializar seu novo teste Sofia® 2 SARS Antigen+ FIA. O Sofia 2 SARS Antigen+ FIA é o primeiro teste rápido de antígeno para detecção da COVID-19 a receber autorização de comercialização da FDA.
- Em março de 2023, o teste LumiraDx SARS-CoV-2 Ag foi autorizado para uso em locais de atendimento ao paciente que operam sob um Certificado de Isenção, Certificado de Conformidade ou Certificado de Acreditação da CLIA. Este teste destina-se ao uso por profissionais médicos ou operadores que sejam proficientes na realização de testes em locais de atendimento.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.