Global Electronic Clinical Outcome Assessment Ecoa Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
1.70 Billion
USD
5.52 Billion
2024
2032
USD
1.70 Billion
USD
5.52 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 5.52 Billion | |
|
|
|
|
Segmentação do mercado global de Avaliação Eletrônica de Resultados Clínicos (eCOA), por tipo (Avaliação de Resultados Relatada pelo Médico (CLINRO), Avaliação de Resultados Relatada pelo Paciente (PRO), Avaliação de Resultados Relatada pelo Observador (OBSRO) e Avaliação de Resultados de Desempenho (PERFO)), modalidade (soluções baseadas em site, soluções web e dispositivos portáteis), usuário final (organizações de pesquisa contratadas (CROs), empresas farmacêuticas e de biotecnologia, empresas de dispositivos médicos, hospitais/prestadores de serviços de saúde, empresas de serviços de consultoria, institutos acadêmicos e de pesquisa e outros), modo de entrega (baseado em nuvem e hospedado na web) - Tendências do setor e previsão para 2032
Tamanho do mercado de avaliação eletrônica de resultados clínicos (eCOA)
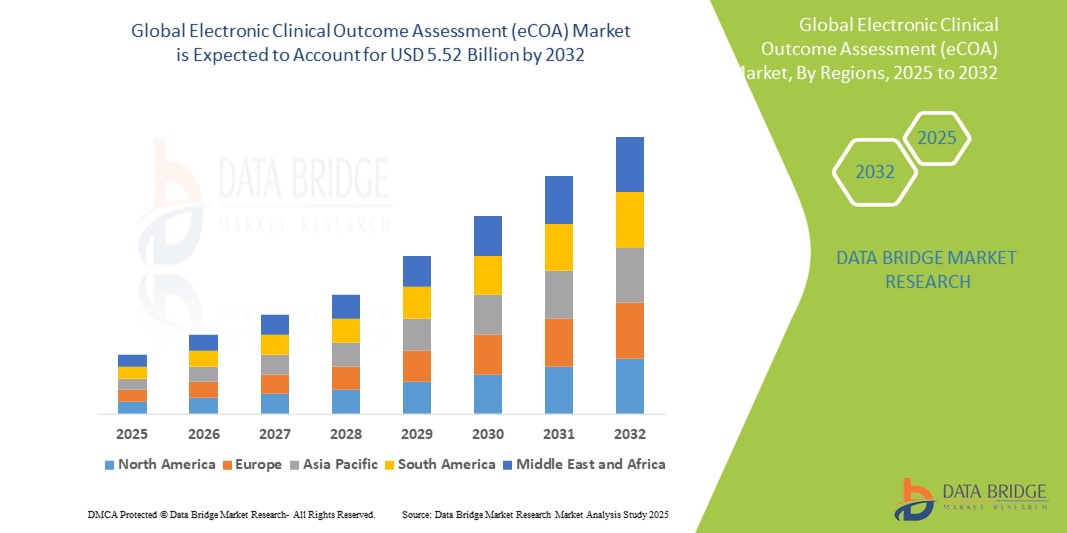
- O tamanho do mercado global de avaliação eletrônica de resultados clínicos (eCOA) foi avaliado em US$ 1,70 bilhão em 2024 e deve atingir US$ 5,52 bilhões até 2032 , com um CAGR de 15,80% durante o período previsto.
- O crescimento do mercado é impulsionado principalmente pela crescente adoção de tecnologias digitais em ensaios clínicos e pesquisas em saúde, facilitando a coleta e o monitoramento de dados de pacientes mais precisos e eficientes.
- Além disso, a crescente demanda por insights de pacientes em tempo real, melhor conformidade regulatória e integridade de dados aprimorada está impulsionando a adoção de soluções eCOA em empresas farmacêuticas, organizações de pesquisa contratadas (CROs) e provedores de saúde.
Análise de Mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA)
- As soluções eCOA, que permitem a captura eletrônica de dados de resultados clínicos diretamente de pacientes, cuidadores ou médicos, são componentes cada vez mais vitais dos ensaios clínicos modernos e da pesquisa em saúde devido à sua precisão de dados aprimorada, recursos de monitoramento em tempo real e integração perfeita com ecossistemas de saúde digital.
- A crescente demanda por eCOA é alimentada principalmente pela ampla adoção de tecnologias de saúde digitais, ênfase crescente em ensaios centrados no paciente e uma preferência crescente por métodos de coleta de dados remotos e fáceis de usar que melhoram a eficiência e a conformidade dos ensaios.
- A América do Norte domina o mercado de avaliação eletrônica de resultados clínicos (eCOA) com a maior participação na receita de 43,5% em 2024, caracterizada pela adoção antecipada de soluções de ensaios clínicos digitais, fortes setores farmacêuticos e de biotecnologia e estruturas regulatórias que dão suporte à captura eletrônica de dados, com os EUA experimentando um crescimento substancial impulsionado por inovações de fornecedores estabelecidos e provedores de tecnologia emergentes com foco em plataformas móveis e baseadas em nuvem
- Espera-se que a Ásia-Pacífico seja a região de crescimento mais rápido no mercado de avaliação eletrônica de resultados clínicos (eCOA) durante o período previsto devido ao aumento das atividades de ensaios clínicos, ao aumento dos investimentos em saúde e à crescente conscientização sobre os benefícios das ferramentas digitais em mercados emergentes como China e Índia.
- O segmento de Avaliação de Resultados Relatados pelo Paciente (PRO) domina o mercado de avaliação eletrônica de resultados clínicos (eCOA) com uma participação de mercado de 48,5% em 2024, impulsionado por seu papel crítico na captura das perspectivas dos pacientes sobre a eficácia do tratamento e a qualidade de vida, que são cada vez mais priorizadas por patrocinadores e agências reguladoras.
Escopo do Relatório e Segmentação do Mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA)
|
Atributos |
Principais insights de mercado sobre Avaliação Eletrônica de Resultados Clínicos (eCOA) |
|
Segmentos abrangidos |
|
|
Países abrangidos |
América do Norte
Europa
Ásia-Pacífico
Oriente Médio e África
Ámérica do Sul
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, análises de preços, análises de participação de marca, pesquisas com consumidores, análises demográficas, análises da cadeia de suprimentos, análises da cadeia de valor, visão geral de matérias-primas/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências de mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA)
“Eficiência aprimorada em ensaios clínicos por meio de IA e monitoramento remoto de pacientes”
- Uma tendência significativa e crescente no mercado global de eCOA é o aprofundamento da integração de tecnologias de inteligência artificial (IA) e monitoramento remoto de pacientes em plataformas de coleta de dados de ensaios clínicos. Essa fusão de tecnologias está aprimorando significativamente a precisão, a pontualidade e a centralidade no paciente das avaliações de resultados clínicos.
- Por exemplo, os principais provedores de eCOA, como Medidata e ERT, incorporam análises baseadas em IA para identificar padrões em dados relatados por pacientes, permitindo a detecção precoce de eventos adversos e a melhoria da tomada de decisões em ensaios clínicos. Da mesma forma, dispositivos vestíveis combinados com plataformas eCOA facilitam o monitoramento contínuo em tempo real das métricas de saúde do paciente, além das visitas tradicionais ao local.
- A integração de IA no eCOA possibilita recursos como análise preditiva para adesão do paciente, verificações automatizadas da qualidade dos dados e alertas inteligentes para respostas incomuns do paciente. Além disso, os recursos de monitoramento remoto oferecem aos pacientes interfaces convenientes e fáceis de usar para relatar os resultados em casa, melhorando a integridade e o engajamento dos dados.
- A integração perfeita dos sistemas eCOA com plataformas mais amplas de saúde digital e gestão de ensaios clínicos permite que os patrocinadores centralizem a gestão de dados e otimizem os fluxos de trabalho dos ensaios. Por meio de painéis unificados, as equipes clínicas podem monitorar os dados dos pacientes, o desempenho do local e a conformidade regulatória em tempo real.
- Essa tendência em direção a soluções de resultados clínicos mais inteligentes, conectadas e amigáveis ao paciente está reformulando fundamentalmente as expectativas em relação à captura de dados de ensaios clínicos. Consequentemente, empresas como a Oracle Health e a CRF Health estão desenvolvendo plataformas eCOA habilitadas para IA com recursos preditivos aprimorados e funcionalidades de captura remota de dados.
- A demanda por soluções eCOA com integração de IA e monitoramento remoto de pacientes está crescendo rapidamente nos setores farmacêutico, de biotecnologia e de dispositivos médicos, à medida que as partes interessadas priorizam cada vez mais a eficiência dos testes, a precisão dos dados e a experiência do paciente.
Dinâmica de mercado de avaliação eletrônica de resultados clínicos (eCOA)
Motorista
“Crescente demanda por ensaios clínicos centrados no paciente e precisão de dados digitais”
- O foco crescente em ensaios clínicos centrados no paciente, combinado com a crescente necessidade de coleta de dados digitais precisos e em tempo real, é um fator significativo para a crescente demanda por soluções de avaliação eletrônica de resultados clínicos (eCOA).
- Por exemplo, em janeiro de 2024, a Medidata, uma empresa da Dassault Systèmes, introduziu novos aprimoramentos baseados em IA em sua plataforma eCOA para melhorar a adesão dos pacientes e a qualidade dos dados em ensaios descentralizados. Espera-se que tais inovações, por parte de importantes players do setor, impulsionem o crescimento do mercado de eCOA durante o período previsto.
- À medida que as empresas farmacêuticas e de biotecnologia buscam otimizar os processos de ensaios clínicos e reduzir o tempo de colocação no mercado, as plataformas eCOA oferecem recursos avançados, como captura de dados em tempo real, relatórios remotos de pacientes e validação automatizada, proporcionando uma melhoria substancial em relação aos métodos tradicionais baseados em papel.
- Além disso, a crescente adoção de modelos de ensaios clínicos descentralizados e híbridos está posicionando o eCOA como um componente essencial para a coleta remota de dados, melhorando o envolvimento do paciente e mantendo altos padrões de conformidade regulatória.
- A capacidade das plataformas eCOA de aumentar a eficiência dos ensaios clínicos por meio da coleta eletrônica de dados, suporte multilíngue e integração com dispositivos vestíveis ou aplicativos móveis é um fator-chave que impulsiona sua adoção por CROs, empresas farmacêuticas e instituições de pesquisa. A crescente ênfase na redução das taxas de abandono de ensaios clínicos e na melhoria da integridade dos dados reforça ainda mais a ampla integração das soluções eCOA na pesquisa clínica moderna.
Restrição/Desafio
“Preocupações com a privacidade de dados, conformidade regulatória e altos custos de implementação”
- As preocupações em torno da privacidade de dados, da conformidade regulatória e dos altos custos iniciais de implementação de plataformas de avaliação eletrônica de resultados clínicos (eCOA) representam desafios significativos para uma adoção mais ampla pelo mercado
- Como os sistemas eCOA envolvem a captura e transmissão eletrônica de dados confidenciais de saúde do paciente, eles estão sujeitos a regulamentações rígidas de proteção de dados, como HIPAA, GDPR e 21 CFR Parte 11, tornando a conformidade complexa e exigindo muitos recursos para patrocinadores e CROs.
- Por exemplo, vários patrocinadores de ensaios clínicos expressaram cautela na transição completa para sistemas eCOA devido às incertezas em torno das regras de localização de dados e à complexidade de garantir a conformidade de dados transfronteiriços, especialmente em ensaios multirregionais.
- Enfrentar esses desafios exige uma infraestrutura robusta de segurança de dados, auditorias regulares e adesão aos padrões globais de conformidade. Os principais provedores de eCOA, como Oracle Health e Signant Health, investem significativamente em plataformas criptografadas e treinamento regulatório para mitigar riscos e manter a confiança das partes interessadas nos ensaios clínicos.
- Além disso, o alto custo inicial associado à implantação de sistemas eCOA — incluindo taxas de licenciamento, aquisição de hardware, treinamento de equipe e integração de sistemas — pode ser uma barreira à entrada, especialmente para organizações de pesquisa de pequeno e médio porte. Embora os benefícios a longo prazo, como maior precisão dos dados e redução da duração dos ensaios, sejam amplamente reconhecidos, o ônus financeiro inicial pode limitar a adoção entre instituições com recursos limitados.
- Superar esses desafios por meio de modelos de preços escaláveis, entrega baseada em nuvem e inovação contínua em plataformas seguras e fáceis de usar será essencial para impulsionar uma adoção mais ampla e sustentada de soluções eCOA em todo o cenário de pesquisa clínica.
Escopo de mercado da Avaliação Eletrônica de Resultados Clínicos (eCOA)
O mercado é segmentado com base no tipo, modalidade, usuário final e modo de entrega
- Por tipo
Com base no tipo, o mercado de avaliação eletrônica de resultados clínicos (eCOA) é segmentado em resultados relatados pelo paciente (PRO), resultados relatados pelo clínico (ClinRO), resultados relatados pelo observador (ObsRO) e resultados de desempenho (PerfO). O segmento de resultados relatados pelo paciente (PRO) deteve a maior participação de mercado na receita, com 48,5% em 2024, impulsionado por sua abordagem centrada no paciente, que captura insights em primeira mão sobre as experiências, sintomas e resultados de tratamento dos pacientes. As ferramentas PRO permitem que os pacientes relatem seus dados de saúde diretamente em tempo real por meio de plataformas eletrônicas, aumentando a precisão dos dados e o envolvimento do paciente, melhorando, em última análise, a qualidade dos estudos clínicos.
Prevê-se que o segmento de resultados relatados por clínicos (ClinRO) apresentará um crescimento substancial durante o período previsto, devido à crescente complexidade dos ensaios clínicos e à necessidade de métodos de coleta de dados precisos e padronizados. O ClinRO envolve avaliações realizadas por profissionais de saúde treinados, fornecendo dados objetivos e confiáveis para avaliar intervenções clínicas, especialmente nos casos em que o autorrelato do paciente não é viável.
- Por Modalidade
Com base na modalidade, o mercado de avaliação eletrônica de resultados clínicos (eCOA) é segmentado em soluções baseadas em site, soluções web e dispositivos portáteis. O segmento de soluções web deteve a maior fatia de mercado em 2024, devido às suas interfaces amigáveis, fácil acessibilidade e menor necessidade de investimento. Soluções hospedadas na web armazenam dados do cliente em servidores em nuvem, acessíveis via web com hardware básico e conexão à internet, oferecendo flexibilidade para personalização e permitindo a adaptação às necessidades específicas do cliente.
Espera-se que o segmento de dispositivos portáteis apresente um crescimento significativo durante o período previsto, impulsionado pela crescente adoção de tecnologias móveis em ensaios clínicos. Dispositivos portáteis facilitam a captura de dados em tempo real e melhoram a adesão dos pacientes, tornando-os uma opção atraente para estudos clínicos descentralizados e remotos.
- Por usuário final
Com base no usuário final, o mercado de avaliação eletrônica de resultados clínicos (eCOA) é segmentado em empresas farmacêuticas e de biotecnologia, organizações de pesquisa contratadas (CROs), empresas de dispositivos médicos, hospitais/prestadores de serviços de saúde, empresas de serviços de consultoria, institutos acadêmicos e de pesquisa, entre outros. O segmento de empresas farmacêuticas e de biotecnologia domina o mercado, respondendo por 50,66% do mercado em 2024. Essa dominância se deve ao papel fundamental que as soluções de eCOA desempenham na otimização da coleta e análise de dados durante os processos de desenvolvimento de medicamentos, garantindo a conformidade com os padrões regulatórios e aumentando a precisão dos dados de ensaios clínicos.
Prevê-se que o segmento de organizações de pesquisa contratadas (CROs) testemunhe um crescimento substancial durante o período previsto, impulsionado pela tendência crescente de grandes empresas biofarmacêuticas e de dispositivos médicos terceirizarem a gestão de pesquisas clínicas. As CROs oferecem serviços abrangentes que abrangem o desenho de estudos, o recrutamento de pacientes, a coleta e a análise de dados, tornando-as participantes essenciais no cenário de eCOA.
Por modo de entrega
Com base no modo de entrega, o mercado de avaliação eletrônica de resultados clínicos (eCOA) é segmentado em soluções baseadas em nuvem e hospedadas na web. O segmento de soluções hospedadas na web detinha a maior participação de mercado, 58,9% em 2025, devido à sua relação custo-benefício em relação às soluções baseadas em nuvem. Plataformas hospedadas na web envolvem menores investimentos iniciais em infraestrutura para os usuários finais, reduzindo os gastos de capital para empresas farmacêuticas, CROs e provedores de saúde.
Espera-se que o segmento de soluções baseadas em nuvem testemunhe um crescimento significativo durante o período previsto, impulsionado por sua escalabilidade, flexibilidade e eficiência de custos. Plataformas baseadas em nuvem facilitam e agilizam o acesso aos dados para as partes interessadas em ensaios clínicos, independentemente de sua localização, o que é crucial para ensaios em múltiplos locais.
Análise regional do mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA)
- A América do Norte domina o mercado de avaliação eletrônica de resultados clínicos (eCOA) com a maior participação na receita de 43,5% em 2024, impulsionada pela adoção antecipada de soluções de ensaios clínicos digitais, fortes setores farmacêuticos e de biotecnologia e estruturas regulatórias que dão suporte à captura eletrônica de dados.
- A região se beneficia de uma estrutura regulatória robusta que apoia a transformação digital da pesquisa clínica, incentivando empresas farmacêuticas e organizações de pesquisa contratadas a adotar plataformas eCOA para melhorar a precisão dos dados e a conformidade regulatória.
- Além disso, fortes investimentos em P&D, infraestrutura de saúde consolidada e adoção antecipada de ensaios clínicos descentralizados e centrados no paciente contribuem significativamente para o crescimento do mercado. A presença de provedores líderes de soluções de eCOA e CROs acelera ainda mais a expansão regional de ferramentas eletrônicas de avaliação de resultados clínicos.
Visão do mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA) dos EUA
O mercado americano de avaliação eletrônica de resultados clínicos (eCOA) capturou a maior fatia da receita, de 79,6%, em 2024, na América do Norte, impulsionado pela liderança do país em ensaios clínicos e pela rápida digitalização das práticas de pesquisa clínica. Agências reguladoras como a FDA defendem fortemente o uso de ferramentas digitais para melhorar a qualidade dos dados e o engajamento dos pacientes, contribuindo para a ampla adoção de sistemas de eCOA. Além disso, a crescente necessidade de modelos de ensaios clínicos descentralizados e híbridos está alimentando a demanda por plataformas remotas e em tempo real para coleta de dados de pacientes. O mercado americano também se beneficia de um forte financiamento em P&D, da grande presença de gigantes farmacêuticas e de uma sofisticada infraestrutura de TI em saúde.
Visão do mercado europeu de Avaliação Eletrônica de Resultados Clínicos (eCOA)
O mercado europeu de avaliação eletrônica de resultados clínicos (eCOA) deverá crescer a uma taxa composta de crescimento anual (CAGR) substancial ao longo do período previsto, impulsionado pela crescente ênfase regulatória em evidências do mundo real, foco no paciente e padronização de dados em todos os ensaios clínicos. A crescente necessidade de soluções digitais multilíngues e culturalmente adaptadas em toda a UE está acelerando a adoção de plataformas de eCOA flexíveis e escaláveis. Além disso, o aumento das colaborações em pesquisa acadêmica, aliado a políticas favoráveis à transformação digital da saúde, impulsiona o crescimento regional. Países como Alemanha, Reino Unido e França lideram a adoção de tecnologias em seus respectivos ecossistemas de ensaios clínicos.
Visão do mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA) do Reino Unido
Prevê-se que o mercado de avaliação eletrônica de resultados clínicos (eCOA) do Reino Unido cresça a um CAGR considerável durante o período previsto, impulsionado por seu forte setor de P&D biofarmacêutico e pelas estratégias proativas de saúde digital do NHS. O número crescente de ensaios clínicos descentralizados e a clareza regulatória em torno da captura eletrônica de dados estão impulsionando a adoção pelo mercado. Com um cenário avançado de pesquisa clínica e investimentos significativos em informática em saúde, o Reino Unido está testemunhando uma rápida adoção das tecnologias de eCOA para garantir a conformidade, melhorar o engajamento do paciente e permitir o rastreamento eficiente de resultados.
Visão do mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA) na Alemanha
Espera-se que o mercado alemão de avaliação eletrônica de resultados clínicos (eCOA) se expanda a um CAGR considerável durante o período previsto, impulsionado pela reputação do país em excelência em ensaios clínicos e pelas rigorosas leis de proteção de dados. Os órgãos reguladores alemães enfatizam a confiabilidade e a segurança dos dados clínicos, incentivando patrocinadores e CROs a investir em soluções de eCOA seguras e validadas. Além disso, a crescente demanda da Alemanha por captura de dados em tempo real em ensaios de fase I a IV e sua sólida infraestrutura de TI para a área da saúde promovem uma maior integração das tecnologias de eCOA na pesquisa de dispositivos médicos e farmacêutica.
Visão do mercado de avaliação eletrônica de resultados clínicos (eCOA) da Ásia-Pacífico
O mercado de avaliação eletrônica de resultados clínicos (eCOA) da Ásia-Pacífico deverá crescer com a CAGR mais rápida durante o período previsto de 2025 a 2032, impulsionado pelo aumento da atividade de pesquisa clínica e pela transformação digital da saúde em países como China, Índia, Coreia do Sul e Japão. A expansão de ensaios clínicos multinacionais e a disponibilidade de populações de pacientes diversificadas impulsionam o crescimento regional. Os incentivos governamentais para a adoção de plataformas digitais de saúde e a crescente demanda por soluções móveis tornam a adoção do eCOA mais viável e disseminada em regiões urbanas e semiurbanas. Parcerias locais entre CROs e empresas farmacêuticas globais estão impulsionando ainda mais a implementação do eCOA.
Visão do mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA) do Japão
O mercado japonês de avaliação eletrônica de resultados clínicos (eCOA) está ganhando força devido à sofisticação tecnológica do país, ao envelhecimento da população e à ênfase em dados de qualidade em ensaios clínicos. O órgão regulador japonês, o PMDA, está cada vez mais receptivo a endpoints digitais e ferramentas de captura remota de dados. O mercado também está sendo moldado pelo aumento de ensaios clínicos domiciliares e ambulatoriais, impulsionando a necessidade de sistemas de eCOA precisos e amigáveis ao paciente. Espera-se que a integração com ecossistemas eClinical mais amplos e ferramentas de engajamento do paciente baseadas em IA acelerem ainda mais o crescimento.
Visão do mercado de Avaliação Eletrônica de Resultados Clínicos (eCOA) da Índia
O mercado indiano de avaliação eletrônica de resultados clínicos (eCOA) foi responsável pela maior fatia da receita de mercado na região Ásia-Pacífico em 2024, impulsionado por um aumento na atividade de ensaios clínicos, uma população com conhecimento tecnológico e a expansão da capacidade de fabricação farmacêutica. O cenário de CROs (Centro de Avaliação de Resultados) econômico da Índia e as políticas governamentais favoráveis à digitalização da saúde estão incentivando patrocinadores globais a implementar ferramentas de eCOA em ensaios clínicos nacionais. A crescente penetração de smartphones, o crescimento da telemedicina e a melhoria da conectividade à internet em áreas urbanas e semiurbanas estão tornando as plataformas de eCOA baseadas em dispositivos móveis e hospedadas na nuvem mais acessíveis e amplamente adotadas.
Participação de mercado da Avaliação Eletrônica de Resultados Clínicos (eCOA)
O setor de avaliação eletrônica de resultados clínicos (eCOA) é liderado principalmente por empresas bem estabelecidas, incluindo:
- IQVIA (EUA)
- Clario (EUA)
- Medidata (EUA)
- Veeva Systems (EUA)
- Tecnologia de Recursos Terrestres (EUA)
- Oracle Health Sciences (EUA)
- YPrime, LLC (EUA)
- ArisGlobal LLC (EUA)
- Castor EDC (Holanda)
- eClinicalWorks (EUA)
- Medrio, Inc. (EUA)
- ClinOne (EUA)
- Signant Health (EUA)
- Clinical Ink, Inc. (EUA)
- Curebase, Inc. (EUA)
- Kayentis (França)
- Calyx (Reino Unido)
- Datacubed Health (EUA)
- HealthDiary, Inc. (EUA)
Últimos desenvolvimentos no mercado global de avaliação eletrônica de resultados clínicos (eCOA)
- Em maio de 2025, a Clario (EUA) adquiriu a unidade de eCOA da WCG Clinical (EUA), um movimento estratégico para reforçar sua liderança em soluções de dados de endpoint digitais, especialmente para ensaios clínicos em neurociência. Esta aquisição expande a plataforma abrangente de dados de endpoint da Clario, permitindo melhor suporte para ambientes de ensaios complexos e consolidando ainda mais sua posição no cenário de eCOA em rápida evolução.
- Em maio de 2025, o Critical Path Institute (EUA) deu continuidade à sua iniciativa "eCOA: Getting Better Together", com o objetivo de unificar patrocinadores, fornecedores de tecnologia e reguladores. Este esforço colaborativo, que se estenderá até março de 2025, concentra-se no estabelecimento de melhores práticas pré-competitivas e em um léxico comum para a captura de dados do eCOA, promovendo a padronização e acelerando a adoção em diversas regiões.
- Em novembro de 2023, a Clinical Ink aprimorou seu pacote de engajamento de pacientes incorporando a ferramenta de diagnóstico comportamental SPUR da Observia. Essa integração combina avaliação comportamental com modificação do estilo de vida, eCOA, eSource e biomarcadores digitais, com o objetivo de proporcionar uma compreensão mais holística do comportamento do paciente e aprimorar os resultados dos ensaios clínicos.
- Em outubro de 2023, a Clario firmou uma parceria estratégica com a Trial Data, uma prestadora de serviços de ensaios clínicos descentralizados (DCT). Essa colaboração fortalece a presença da Clario no cenário de ensaios clínicos da China, combinando sua expertise para fornecer soluções de ensaios descentralizados de última geração e promover abordagens centradas no paciente na região.
- Em dezembro de 2022, a Suvoda LLC, uma empresa de tecnologia para ensaios clínicos de eCOA, lançou seu kit de ferramentas para design de avaliações eletrônicas de resultados clínicos (eCOA). Este kit foi criado para se integrar perfeitamente ao Suvoda IRT e ao eConsent, abordando inadequações históricas na implementação de eCOA e visando otimizar o processo de design.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.