Global Denosumab Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
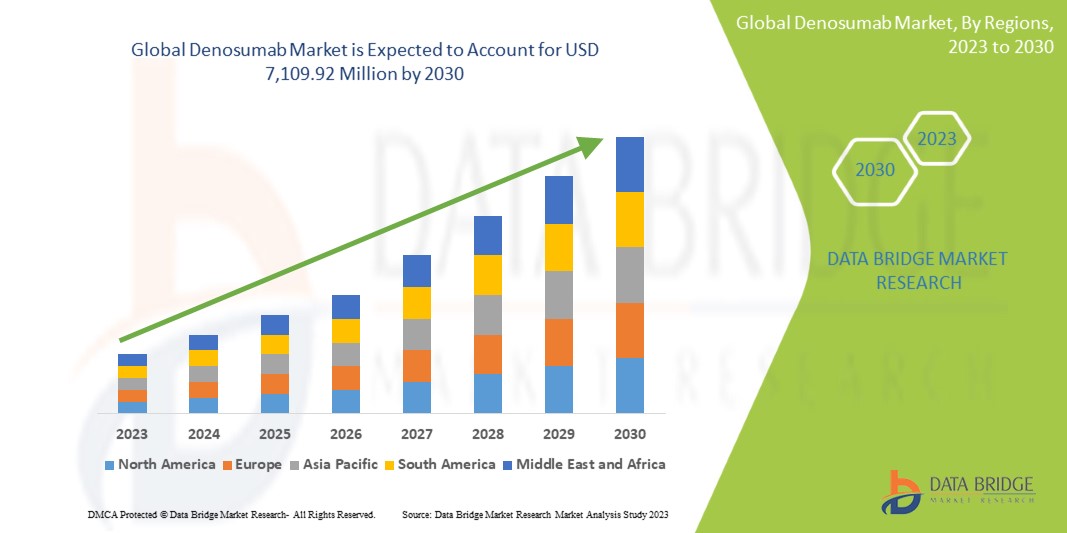
2,892.17 Million
USD
7,109.92 Million
2022
2030
USD
2,892.17 Million
USD
7,109.92 Million
2022
2030
| 2023 –2030 | |
| USD 2,892.17 Million | |
| USD 7,109.92 Million | |
|
|
|
|
Mercado global de denosumabe, por classificação de medicamentos (Prolia, Xgeva e outros), utilizadores finais (hospitais, cuidados domiciliários, clínicas especializadas, centros de cirurgia ambulatória e outros), canal de distribuição (farmácia hospitalar, farmácia de retalho e farmácia online ) – indústria Tendências e previsões para 2030.
Análise e dimensão do mercado de Denosumab
A incidência da osteoporose tende a aumentar com a idade, principalmente nas mulheres na pós-menopausa, devido às alterações hormonais que ocorrem durante a menopausa. No entanto, a osteoporose também pode afetar homens e indivíduos mais jovens. De acordo com a Fundação Internacional de Osteoporose (IOF), estima-se que 200 milhões de mulheres em todo o mundo tenham osteoporose. Além disso, aproximadamente 1 em cada 3 mulheres com mais de 50 anos e 1 em cada 5 homens com mais de 50 anos sofrerão fraturas osteoporóticas ao longo da vida.
A Data Bridge Market Research analisa que o mercado global de denosumabe, que era de 2.892,17 milhões de dólares em 2022, dispararia para 7.109,92 milhões de dólares até 2030, e deverá sofrer um CAGR de 11,9% durante o período previsto . Este indica o valor de mercado. “Prolia” domina o segmento de classificação de medicamentos do mercado global de denosumabe devido à crescente procura de um melhor tratamento para as doenças ósseas.
Para além dos insights sobre os cenários de mercado, tais como o valor de mercado, a taxa de crescimento, a segmentação, a cobertura geográfica e os principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research incluem também análises aprofundadas de especialistas, epidemiologia dos doentes, análise de pipeline, análise de preços, e quadro regulamentar.
Âmbito do Relatório e Segmentação de Mercado
|
Métrica de Reporte |
Detalhes |
|
Período de previsão |
2023 a 2030 |
|
Ano base |
2022 |
|
Anos históricos |
2021 (Personalizável para 2015-2020) |
|
Unidades quantitativas |
Receita em milhões de dólares americanos, volumes em unidades, preços em dólares americanos |
|
Segmentos abrangidos |
Classificação dos medicamentos (Prolia, Xgeva e outros), utilizadores finais (hospitais, cuidados domiciliários, clínicas especializadas, centros de cirurgia ambulatória e outros), canal de distribuição (farmácia hospitalar, farmácia de retalho e farmácia on-line) |
|
Países abrangidos |
EUA, Canadá e México na América do Norte, Alemanha, França, Reino Unido, Holanda, Suíça, Bélgica, Rússia, Itália, Espanha, Turquia, Resto da Europa na Europa, China, Japão, Índia, Coreia do Sul, Singapura, Malásia , Austrália, Tailândia, Indonésia, Filipinas, Resto da Ásia-Pacífico, Arábia Saudita, Emirados Árabes Unidos, África do Sul, Egito, Israel, Resto do Médio Oriente e África, Brasil, Argentina e Resto da América do Sul |
|
Atores do mercado abrangidos |
Merck & Co., Inc (EUA), Amneal Pharmaceutical Inc (EUA), Teva Pharmaceutical Industries Ltd (Israel), Zydus Cadila (Índia), Novartis Ag (Suíça), Pfizer Inc (EUA), Astrazeneca (Reino Unido), Glaxosmithkline Plc (Reino Unido), Bristol Myers Squibb Company (EUA), Johnson & Johnson Service Inc (EUA), F. Hoffmann- La Roche Ltd (Suíça), Bayer AG (Alemanha), Amgen Inc (EUA), Sanofi (França) , Biogen (EUA), Regeneron Pharmaceuticals Inc (EUA), Eli Lilly the Company (EUA), AbbiVie Inc (EUA), Mylan NV (EUA) e Eisai Co., Ltd (Japão) |
|
Oportunidades de Mercado |
|
Definição de Mercado
A injeção de denosumabe (Prolia) refere-se ao tipo de medicamento utilizado para tratar a osteoporose, uma condição em que os ossos se tornam finos e fracos e se partem facilmente na população feminina. A condição ocorre sobretudo em mulheres que passaram pela menopausa e têm maior risco de fraturas.
Dinâmica do mercado global de Denosumab
Motoristas
- Aumento da prevalência da osteoporose
A osteoporose é uma condição comum caracterizada pela diminuição da densidade óssea e pelo aumento do risco de fraturas. À medida que a população global envelhece, prevê-se que a prevalência da osteoporose aumente, aumentando a procura de medicamentos como o Denosumab, que podem ajudar a prevenir fracturas e a melhorar a saúde óssea.
- Crescente consciencialização e diagnóstico
Com o aumento da consciencialização sobre a osteoporose e o seu impacto na saúde em geral, mais pessoas procuram diagnóstico e tratamento. Esta crescente consciencialização e diagnóstico contribuem para a procura de medicamentos como o Denosumab.
- Perfil de eficácia e segurança para indicações alargadas
O denosumabe demonstrou eficácia na redução do risco de fraturas e na melhoria da densidade óssea em ensaios clínicos . O seu perfil de segurança, quando utilizado de acordo com as instruções, tem sido geralmente bem tolerado. Estes fatores contribuem para a sua popularidade e adoção no mercado. O denosumabe recebeu aprovações para indicações adicionais para além da osteoporose, como a prevenção de eventos relacionados com o esqueleto em doentes com metástases ósseas de tumores sólidos e o tratamento de tumores ósseos de células gigantes. Estas indicações alargadas ampliaram o mercado para o Denosumab.
Oportunidades
- Proficiência em Investigação e Desenvolvimento
Os esforços contínuos de investigação e desenvolvimento no domínio da saúde óssea oferecem oportunidades para o desenvolvimento de novas terapias e tratamentos combinados. Os fabricantes de Denosumab podem explorar estas oportunidades para otimizar ainda mais a eficácia e a segurança do Denosumab ou desenvolver novos tratamentos que complementem o seu mecanismo de ação.
- Aumento da população idosa
A população global está a envelhecer, com uma maior proporção de idosos. Uma vez que a idade é um fator de risco significativo para a osteoporose e outras condições relacionadas com os ossos, esta tendência demográfica apresenta uma oportunidade substancial para o mercado de Denosumab satisfazer a crescente procura de tratamentos de saúde óssea.
Restrições/Desafios
- Concorrência das terapias existentes
O denosumabe enfrenta a concorrência de outras terapêuticas estabelecidas para o tratamento da osteoporose, como os bifosfonatos e os moduladores seletivos dos recetores de estrogénio. Estas terapias têm sido amplamente utilizadas e têm um longo historial de eficácia e segurança, o que torna difícil para o Denosumab ganhar quota de mercado.
- Elevado custo da terapêutica e tratamento com denosumabe
O denosumab é uma terapia biológica, e os medicamentos biológicos têm geralmente um custo mais elevado quando comparados com os medicamentos tradicionais de pequenas moléculas. O elevado custo do Denosumab pode limitar a sua acessibilidade e acessibilidade para alguns doentes, especialmente em regiões com recursos de saúde limitados ou cobertura de seguro inadequada.
- Dados de segurança e eficácia a longo prazo
Embora o Denosumab tenha demonstrado eficácia e segurança em ensaios clínicos, os dados a longo prazo sobre a sua utilização para além de vários anos são limitados. A disponibilidade de dados de segurança e eficácia a longo prazo é crucial para ganhar a confiança dos profissionais de saúde e dos doentes, especialmente em condições crónicas como a osteoporose.
- Desafios complexos de reembolso
As políticas de reembolso e as negociações de preços com os sistemas de saúde e as seguradoras podem representar desafios para os fabricantes de Denosumab. A disponibilidade de reembolso e cobertura de formulários pode variar entre diferentes regiões, impactando o acesso do paciente e a penetração no mercado.
Este relatório do mercado global de denosumabe fornece detalhes dos novos desenvolvimentos recentes, regulamentos comerciais, análise de importação e exportação, análise de produção, otimização da cadeia de valor, quota de mercado, impacto dos participantes do mercado doméstico e localizado, analisa as oportunidades em termos de bolsas de receitas emergentes, alterações nas regulamentações do mercado, análise estratégica do crescimento do mercado, tamanho do mercado, crescimento do mercado das categorias, nichos de aplicação e dominância, aprovações de produtos, lançamentos de produtos, expansões geográficas, inovações tecnológicas no mercado . Para mais informações sobre o mercado de Denosumab, contacte a Data Bridge Market Research para obter um resumo analítico.
Desenvolvimentos recentes
- Em fevereiro de 2023, a Sandoz anunciou que o pedido de licença de produtos biológicos (BLA) para o seu candidato biossimilar denosumabe para revisão foi aceite pela Food and Drug Administration (FDA) para o tratamento de doentes na pré-menopausa com osteoporose que apresentam um risco aumentado de fraturas.
- Em novembro de 2022, a subsidiária do Luye Pharma Group, Boan Biotech, anunciou que a sua injeção de Denosumab (Boyoubei) obteve aprovação para o lançamento do produto na China pela Administração Nacional de Produtos Médicos (NMPA) para o tratamento de mulheres na pós- menopausa com osteoporose e elevado risco de fraturas.
Âmbito do mercado global de Denosumab
O mercado global de denosumabe é segmentado com base na classificação do medicamento, utilizadores finais e canal de distribuição. O crescimento entre estes segmentos irá ajudá-lo a analisar segmentos de baixo crescimento nos setores e fornecerá aos utilizadores uma visão geral e informações valiosas do mercado para os ajudar a tomar decisões estratégicas para identificar as principais aplicações do mercado.
Classificação de Medicamentos
- Prolia
- Xgeva
- Outros
Utilizadores finais
- Hospitais
- Assistência Domiciliária
- Clínicas especializadas
- Centros Cirúrgicos Ambulatoriais
- Outros
Canal de Distribuição
- Farmácia Hospitalar
- Farmácia de retalho
- Farmácia Online
Análise/Insights Regionais do Mercado Global de Denosumab
O mercado global de denosumabe é analisado, e são fornecidos insights e tendências sobre o tamanho do mercado por país, classificação do medicamento, utilizadores finais e canal de distribuição, conforme referenciado acima.
Os países abrangidos pelo relatório do mercado global de denosumab são os EUA, Canadá e México na América do Norte, Alemanha, França, Reino Unido, Países Baixos, Suíça, Bélgica, Rússia, Itália, Espanha, Turquia, Resto da Europa na Europa, China, Japão, Índia , Coreia do Sul, Singapura, Malásia, Austrália, Tailândia, Indonésia, Filipinas, Resto da Ásia-Pacífico, Arábia Saudita, Emirados Árabes Unidos, África do Sul, Egito, Israel, Resto do Médio Oriente e África, Brasil, Argentina e Resto da América do Sul.
Espera-se que a América do Norte domine o mercado global de denosumabe devido à forte base de instalações de saúde, à forte presença de grandes players no mercado, à extraordinária infraestrutura de saúde e ao grande número de pessoas com distúrbios ósseos, principalmente mulheres.
Prevê-se que a Ásia-Pacífico testemunhe um crescimento significativo durante o período previsto de 2023 a 2030 devido ao aumento das iniciativas governamentais para promover a assistência médica, à crescente consciencialização sobre a saúde entre as pessoas e à crescente procura de tecnologia médica avançada para diagnóstico e tratamento.
A secção do relatório sobre os países também fornece fatores individuais que impactam o mercado e alterações na regulamentação do mercado nacional que impactam as tendências atuais e futuras do mercado. Pontos de dados como a análise da cadeia de valor a montante e a jusante, tendências técnicas e análise das cinco forças de Porter, estudos de caso são alguns dos indicadores utilizados para prever o cenário de mercado para países individuais. Além disso, a presença e disponibilidade de marcas globais e os seus desafios enfrentados devido à grande ou escassa concorrência de marcas locais e nacionais, o impacto das tarifas domésticas e das rotas comerciais são considerados ao fornecer uma análise de previsão dos dados do país.
Crescimento da base instalada da infraestrutura de saúde e penetração de novas tecnologias
O mercado global de denosumab também fornece uma análise de mercado detalhada para o crescimento de cada país em termos de despesas de saúde para equipamentos de capital, base instalada de diferentes tipos de produtos para o mercado global de denosumab, o impacto da tecnologia utilizando curvas de linha de vida e as mudanças nos cenários regulamentares de saúde e o seu impacto no mercado global de denosumabe. O período previsto é 2015-2020.
Análise do panorama competitivo e da quota de mercado global de Denosumab
O panorama competitivo do mercado global de denosumabe fornece detalhes por concorrentes. Os detalhes incluídos são a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em investigação e desenvolvimento, novas iniciativas de mercado, presença global, localizações e instalações de produção, capacidades de produção, pontos fortes e fracos da empresa , lançamento do produto, amplitude e abrangência do produto. Os pontos de dados fornecidos acima estão apenas relacionados com o foco das empresas no mercado global de denosumabe.
Alguns dos principais participantes que operam no mercado global de denosumabe são:
- Merck & Co., Inc (EUA)
- Amneal Pharmaceutical Inc (EUA)
- Teva Pharmaceutical Industries Ltd (Israel)
- Zydus Cadila (Índia)
- Novartis Ag (Suíça)
- Pfizer Inc (EUA)
- Astrazeneca (Reino Unido)
- Glaxosmithkline Plc (Reino Unido)
- Bristol Myers Squibb Company (EUA)
- Johnson & Johnson Service Inc (EUA)
- F. Hoffmann- La Roche Ltd (Suíça)
- Bayer AG (Alemanha)
- Amgen Inc (EUA)
- Sanofi (França)
- Biogen (EUA)
- Regeneron Pharmaceuticals Inc (EUA)
- Eli Lilly the Company (EUA)
- AbbiVie Inc (EUA)
- Mylan NV (EUA)
- Eisai Co., Ltd (Japão)
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.