Mercado da Síndrome de Desconforto Respiratório Agudo (SDRA) na Europa, EUA, China e Japão, por causa (doença do coronavírus 2019 (COVID-19), sépsis, inalação de substâncias nocivas, pneumonia grave e outras), tipo (diagnóstico e tratamento ), via de Administração (Oral, Parentérica e Outras), Utente Final (Hospitais, Clínicas Especializadas, Assistência Médica Domiciliária e Outros), Canal de Distribuição (Concurso Direto, Farmácia Hospitalar, Farmácia de Retalho e Farmácia Online) – Tendências do Setor e Previsão até 2030.
Análise e insights do mercado da Síndrome de Angústia Respiratória Aguda (SDRA) na Europa, EUA, China e Japão
A crescente prevalência de doenças infeciosas e respiratórias, como a COVID-19 e a síndrome de desconforto respiratório agudo, e o amplo foco no desenvolvimento de vacinas e produtos terapêuticos e de diagnóstico para estas condições aumentaram a procura do mercado. O avanço da tecnologia para o fácil fornecimento de produtos e as instalações de fabrico rápidas também contribuem para o crescimento do mercado. Os principais participantes do mercado estão altamente focados em lançamentos e aprovações de produtos durante este período crucial. Além disso, o governo e as entidades reguladoras estão a apoiar os participantes do mercado com a aprovação de produtos devido ao crescente surgimento.
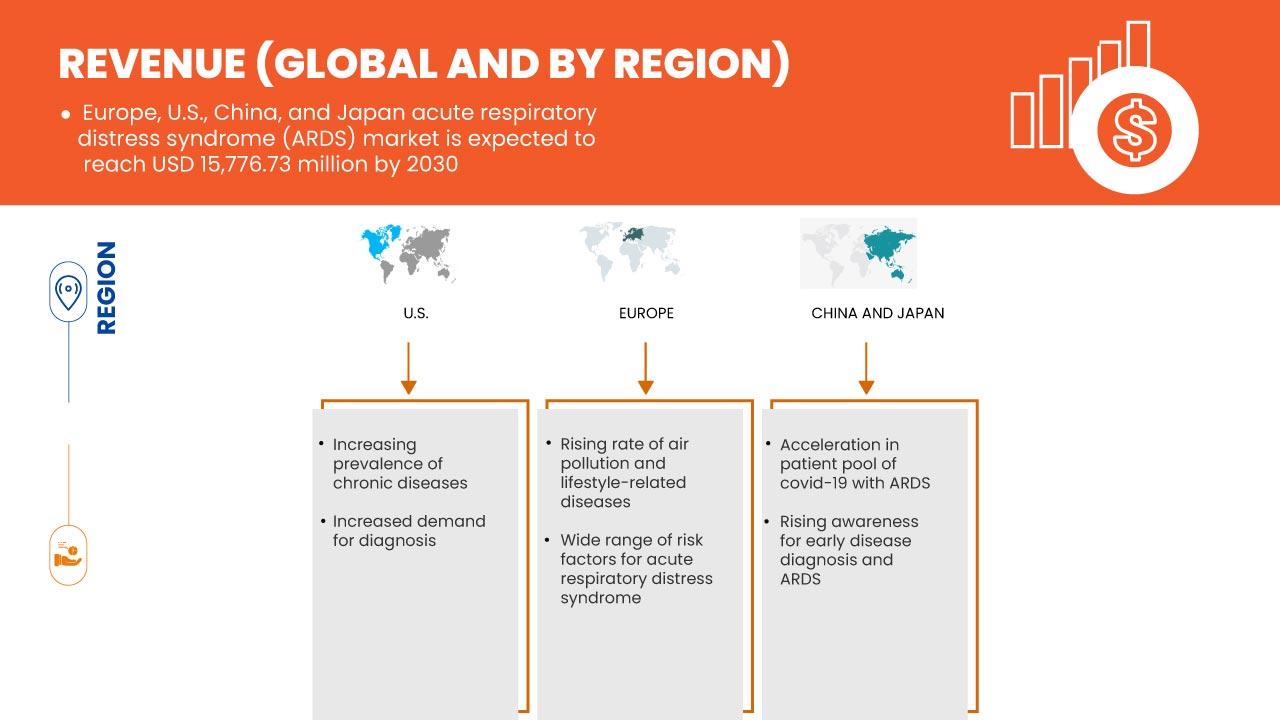
O mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão é favorável e visa reduzir a doença, melhorando assim a recuperação e o desempenho dos indivíduos. A Data Bridge Market Research analisa que o mercado da síndrome respiratória aguda (SDRA) na Europa, EUA, China e Japão crescerá a um CAGR de 9,9% durante o período previsto de 2023 a 2030.
|
Métrica de Reporte |
Detalhes |
|
Período de previsão |
2023 a 2030 |
|
Ano base |
2022 |
|
Anos históricos |
2021 (Personalizável para 2020-2015) |
|
Unidades quantitativas |
Receita em milhões, preço em USD |
|
Segmentos abrangidos |
Por causa (doença do coronavírus 2019 (COVID-19), sépsis, inalação de substâncias nocivas, pneumonia grave e outras), tipo (diagnóstico e tratamento), via de administração (oral, parentérica e outras), utilizador final (hospitais, clínicas especializadas , Assistência Médica Domiciliária e Outros), Canal de Distribuição (Concurso Público, Farmácia Hospitalar, Farmácia de Retalho e Farmácia Online) |
|
País coberto |
EUA, Japão, China, Alemanha, Reino Unido, Itália, França, Espanha, Suíça, Rússia, Turquia, Hungria, Lituânia, Áustria, Irlanda, Noruega, Polónia, Países Baixos e restante Europa |
|
Atores do mercado abrangidos |
Português Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Limited., LivaNova PLC, Gilead Sciences, Inc., Fresenius SE & Co. KGaA, Besmed Health Business Corp., Armstrong Medical, Smiths Medical, ResMed, ALung Technologies, Inc. , Medtronic, F. Hoffmann-La Roche Ltd, Hamilton Medical, Nice Neotech Medical Systems Pvt. Ltd., Pfizer Inc., WEINMANN Emergency Medical Technology GmbH + Co. KG, NIPRO, Terumo Medical Corporation, Getinge AB. |
Definição de Mercado
A síndrome de desconforto respiratório agudo (SDRA) é uma lesão pulmonar com risco de vida que permite a fuga de líquido para os pulmões. A maioria das pessoas que sofrem de SDRA são hospitalizadas por trauma ou doença como a COVID-19. A síndrome ocorre geralmente quando há acumulação de fluidos nos pequenos sacos de ar elásticos dos pulmões, chamados alvéolos. Esta acumulação de líquido faz com que menos oxigénio chegue à corrente sanguínea. Isto priva os órgãos de receber oxigénio suficiente para as suas funções normais. As pessoas com outras doenças desenvolvem SDRA dentro de algumas horas ou dias após a lesão ou infeção precipitante. O risco de morte aumenta com a idade e, dependendo da gravidade da doença, torna-se difícil para os doentes sobreviverem à síndrome. Doenças ou lesões graves que causam danos nas membranas dos pulmões levam à SDRA. As causas subjacentes mais comuns para estas doenças incluem sépsis, inalação de substâncias nocivas, pneumonia grave, lesão na cabeça, no peito ou outra lesão grave, doença do coronavírus 2019 (COVID-19) e outras.
Dinâmica do mercado da Síndrome de Angústia Respiratória Aguda (SDRA) na Europa, EUA, China e Japão
Esta secção trata da compreensão dos impulsionadores, oportunidades, restrições e desafios do mercado. Todos estes são discutidos em detalhe abaixo:
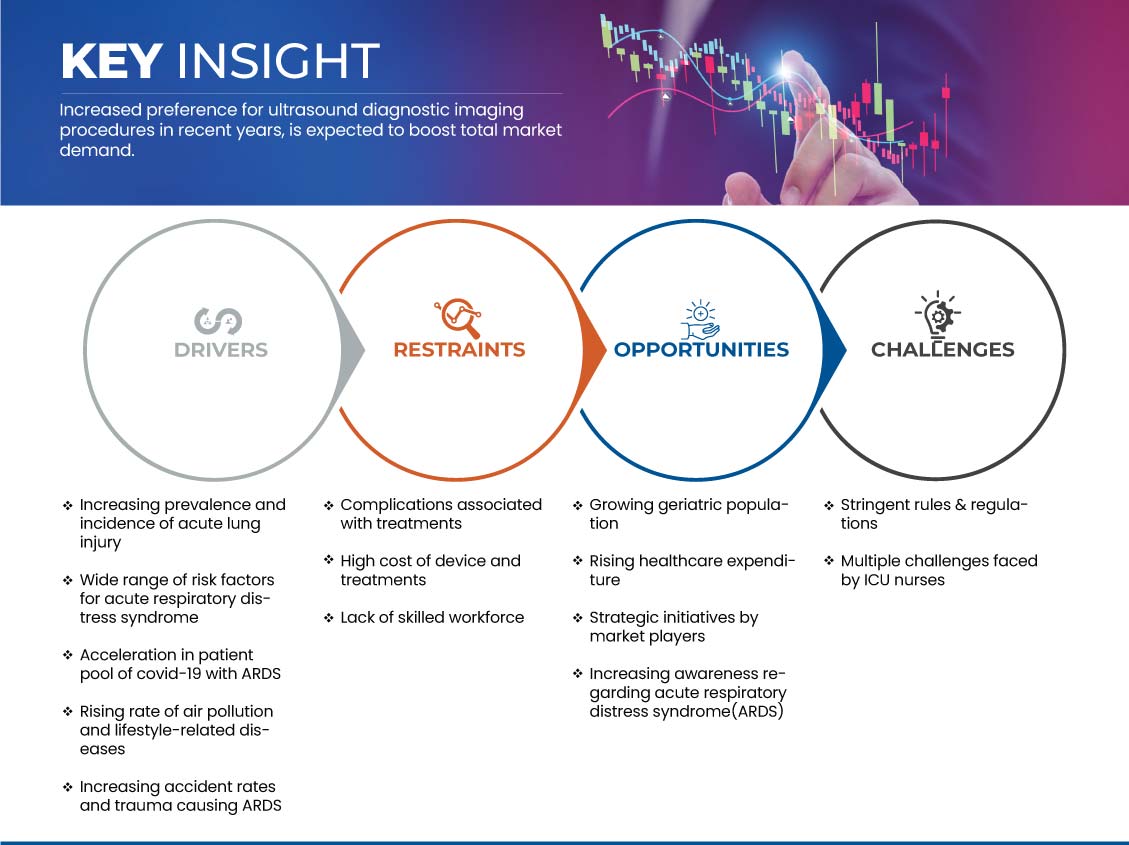
Motoristas
- Aumento da prevalência e incidência de lesão pulmonar aguda
Os doentes com lesões pulmonares agudas estão a ser amplamente relatados devido a vários fatores, como o envelhecimento da população e o número crescente de doentes com sépsis e pneumonia. No entanto, a maioria das pessoas recebe o diagnóstico de lesões pulmonares e síndrome de desconforto respiratório agudo apenas nas fases avançadas. A doença é uma condição de rápida progressão que ocorre em doentes com os pulmões danificados, causando fugas de fluidos corporais. O número de casos de síndrome de desconforto respiratório agudo e de lesões pulmonares está a aumentar devido ao aparecimento de vários vírus causadores de doenças respiratórias nos últimos anos, como a COVID-19.
Desta forma, a incidência e a prevalência da síndrome de desconforto respiratório agudo continuam a aumentar. A doença tem sido amplamente reconhecida como um grande problema clínico em todo o mundo, originando elevada morbilidade e mortalidade. Assim sendo, espera-se que o aumento da prevalência e das taxas de incidência de lesões pulmonares agudas e da síndrome de desconforto respiratório agudo acompanhada impulsione o mercado da síndrome de desconforto respiratório agudo (SDRA) na Europa, EUA, China e Japão.
- Vasta gama de fatores de risco para a Síndrome de Angústia Respiratória Aguda (SDRA)
Existe uma enorme gama de fatores de risco relatados para a síndrome de desconforto respiratório agudo. Existem fatores de risco ambientais e individuais envolvidos na síndrome. Os doentes com SDRA sofrem de vários graus de vasoconstrição da artéria pulmonar, o que causa problemas na obtenção de oxigénio suficiente no sangue. Por isso, geralmente precisam de um ventilador para respirar. A SDRA causa elevada mortalidade e melhora esta condição mortal. A síndrome de sépsis com falência múltipla de órgãos é a causa mais comum de morte, seguida pela insuficiência respiratória. Além disso, a gravidade da SDRA está significativamente associada à taxa de mortalidade entre os doentes críticos com COVID-19.
A SDRA pode ser induzida por múltiplas causas, incluindo trauma. Os fatores de risco para SDRA após trauma múltiplo incluem lesão cerebral traumática e torácica, gravidade e duração do choque, número de hemoderivados transfundidos e cristaloides infundidos.
Oportunidade
- Aumentar a consciencialização sobre a síndrome de angústia respiratória aguda (SARA)
Como a síndrome de desconforto respiratório agudo tem várias causas diferentes, é geralmente ignorada nas causas comuns de morte. A exigência de tratamento técnico avançado e de uma sensibilização adequada para a condição pode diminuir substancialmente a incidência da síndrome de desconforto respiratório agudo. Uma vez que o diagnóstico e a prevenção atempados são cruciais para prevenir ou recuperar mais rapidamente, a atenção do público é muito importante. Os governos e organizações actuais alargaram o âmbito da investigação sobre lesões pulmonares para incluir a prevenção primária da síndrome de desconforto respiratório agudo e reduzir a taxa de morbilidade ou mortalidade da síndrome.
As iniciativas que começaram lá atrás ainda estão a ajudar na prevenção de infeções pulmonares graves, como a SDRA, e apoiam as empresas de biotecnologia e farmacêuticas a inovar a sua investigação para novos avanços nos tratamentos. Embora não exista uma cura adequada ou específica para a SDRA, poucas associações estão a tentar aumentar a consciencialização sobre a síndrome e ajudar os doentes a receberem uma cura atempada para os seus pulmões.
Estes novos programas de iniciativa e unidades de cuidados de suporte iniciados por várias associações de cuidados de saúde e pulmonares estão a sensibilizar as pessoas para a causa e o tratamento adequado da doença atempadamente. Desta forma, aumentar a consciencialização sobre a SDRA através de diversas associações aumenta a oportunidade de crescimento futuro do mercado da síndrome respiratória aguda (SDRA) na Europa, EUA, China e Japão.
Restrição/Desafio
- Elevado custo dos dispositivos e tratamentos
Embora a síndrome de desconforto respiratório agudo esteja a receber uma vasta gama de opções de tratamento avançado, o custo do tratamento mais longo é bastante difícil de pagar para pessoas com rendimentos médios. A utilização de serviços de unidades de tratamento intensivo e de cuidados intensivos está a aumentar em todo o mundo, e o seu elevado custo é uma grande preocupação no actual sistema de saúde. Os doentes com síndrome de desconforto respiratório agudo necessitam, geralmente, de passar por longos internamentos hospitalares com uso frequente de monetarização e ventilação, consumindo uma quantidade significativa de recursos de saúde. Devido a isto, a maioria dos doentes que não podem pagar um internamento de longa duração recebem alta nos estágios iniciais do tratamento. No entanto, isto aumenta as possibilidades e suscetibilidades para novas complicações nas infeções, o que exige recursos de saúde e tratamento adicionais.
Impacto pós-COVID-19 no mercado da Síndrome de Angústia Respiratória Aguda (SDRA) na Europa, EUA, China e Japão
A COVID-19 afetou positivamente o crescimento do mercado, uma vez que se verifica um aumento da procura de síndrome de desconforto respiratório agudo na região. Durante a fase da COVID-19, foi indicado que vários casos são assintomáticos, enquanto 20% dos casos de COVID-19 seguem um curso grave, necessitando de hospitalização. Casos graves da doença COVID-19 acabarão por levar à SDRA e a pneumonia. Foi comprovado que isto é fatal para os indivíduos infetados. Tal como a SDRA demonstra o defeito pulmonar ao danificar os alvéolos, que são pequenos sacos de ar nos pulmões, o mesmo nível de defeito foi observado nos doentes com COVID-19. Isto leva a um influxo repentino de líquido, causando pneumonia. Portanto, a COVID-19 impactou positivamente este mercado.
Desenvolvimentos recentes
- Em maio de 2021, a Medtronic lançou o sistema de monitorização das vias aéreas SonarMed. O sistema utiliza tecnologia acústica para verificar se existe obstrução no tubo endotraqueal. Isto ajudou a empresa a aumentar o seu portfólio de produtos
- Em julho de 2020, a F. Hoffman-La Roche Ltd lançou o teste rápido de anticorpos para SARS-CoV-2. O teste foi lançado em parceria com a SD Biosenseor, Inc. Isto ajudou a empresa a aumentar o seu portefólio de produtos
Âmbito do mercado da Síndrome de Angústia Respiratória Aguda (SDRA) na Europa, EUA, China e Japão
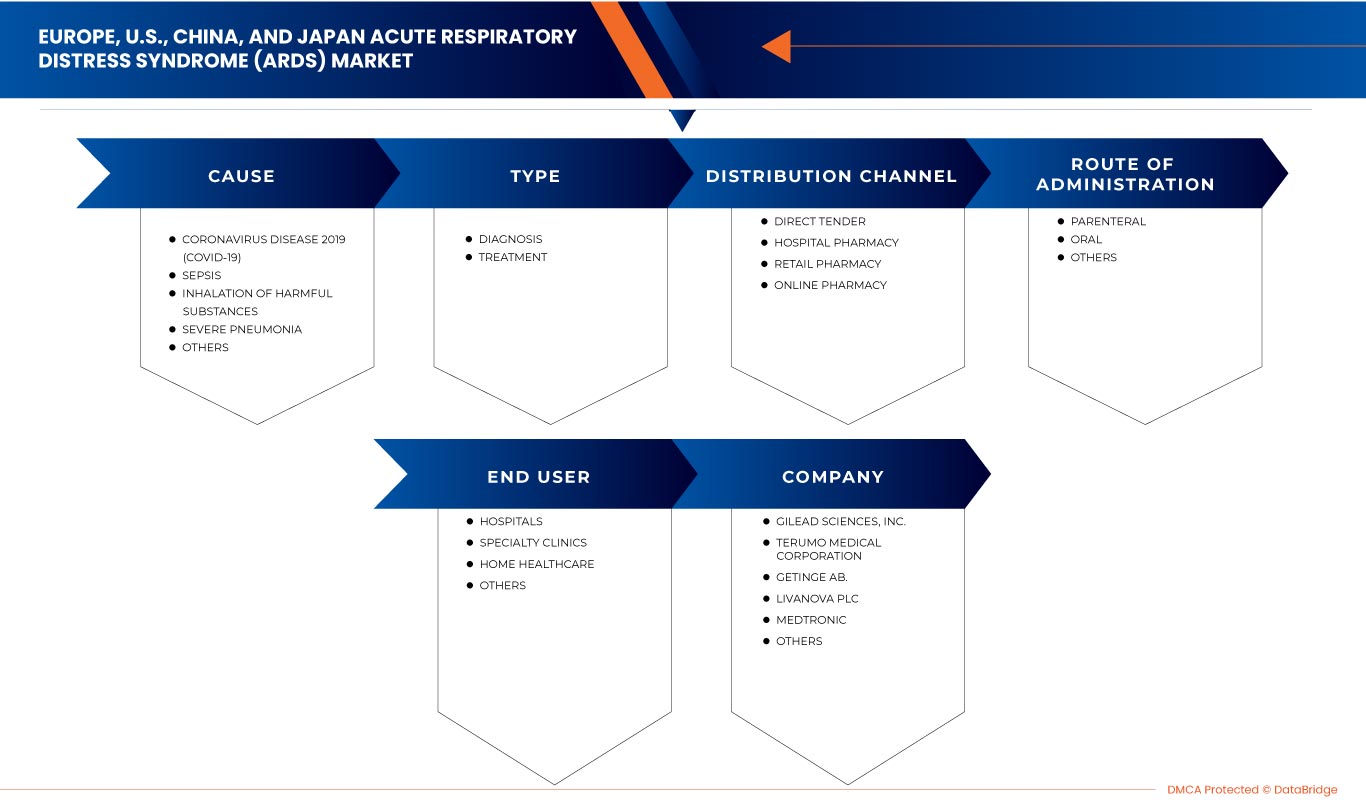
O mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão está categorizado em cinco segmentos com base na causa, tipo, via de administração, utilizador final e canal de distribuição. O crescimento entre segmentos ajuda-o a analisar os nichos de crescimento e as estratégias para abordar o mercado e determinar as suas principais áreas de aplicação e a diferença nos seus mercados-alvo.
Causa
- Doença do Coronavírus 2019 (COVID-19)
- Sepse
- Inalação de substâncias nocivas
- Pneumonia grave
- Outros
Com base na causa, o mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão está segmentado em doença do coronavírus 2019 (COVID-19), sépsis, inalação de substâncias nocivas, pneumonia grave e outros.
Tipo
- Diagnóstico
- Tratamento
Com base no tipo, o mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão está segmentado em diagnóstico e tratamento.
Via de Administração
- Oral
- Parenteral
- Outros
Com base na via de administração, o mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão está segmentado em oral, parentérica e outras.
Utilizador final
- Hospitais
- Clínicas especializadas
- Assistência médica domiciliária
- Outros
Com base no utilizador final, o mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão está segmentado em hospitais, clínicas especializadas, cuidados de saúde domiciliários e outros.
Canal de Distribuição
- Licitação Direta
- Farmácia Hospitalar
- Farmácia de retalho
- Farmácia Online
Com base no canal de distribuição, o mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão está segmentado em licitação direta, farmácia hospitalar, farmácia de retalho e farmácia online.
Análise/Insights do mercado da Síndrome de Angústia Respiratória Aguda (SDRA) na Europa, EUA, China e Japão
O mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão é analisado, e os insights e tendências sobre o tamanho do mercado são fornecidos pela causa, tipo, via de administração, utilizador final e canal de distribuição, conforme referenciado acima .
Os países abrangidos no mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão são os EUA, Japão, China, Alemanha, Reino Unido, Itália, França, Espanha, Suíça, Rússia, Turquia, Hungria, Lituânia, Áustria, Irlanda, Noruega, Polónia, Países Baixos e Resto da Europa.
Espera-se que o mercado da síndrome de desconforto respiratório agudo (SDRA) dos EUA cresça devido ao aumento da prevalência de lesões pulmonares agudas, bem como ao aumento do número de doentes com COVID-19 e SDRA. Estes são os principais fatores que deverão impulsionar o crescimento do mercado no país.
A secção de países do relatório também fornece fatores individuais que impactam o mercado e alterações na regulamentação do mercado que impactam as tendências atuais e futuras do mercado. Pontos de dados como a análise da cadeia de valor a montante e a jusante, tendências técnicas, análise das cinco forças de Porter e estudos de caso são alguns dos indicadores utilizados para prever o cenário de mercado para países individuais. Além disso, a presença e disponibilidade de marcas e os desafios enfrentados devido à grande ou escassa concorrência de marcas locais e nacionais e o impacto das tarifas domésticas e das rotas comerciais são considerados ao fornecer uma análise de previsão dos dados do país.
Análise do panorama competitivo e da quota de mercado da Síndrome de Angústia Respiratória Aguda (SDRA) na Europa, EUA, China e Japão
O panorama competitivo do mercado da síndrome respiratória aguda (SDRA) na Europa, EUA, China e Japão fornece detalhes dos concorrentes. Os detalhes incluídos são a visão geral da empresa, finanças da empresa, receitas geradas, potencial de mercado, investimento em investigação e desenvolvimento, novas iniciativas de mercado, presença, localizações e instalações de produção, capacidades de produção, pontos fortes e fracos da empresa, lançamento do produto, amplitude e abrangência do produto e aplicação. Os pontos de dados fornecidos acima estão apenas relacionados com o foco da empresa no mercado da síndrome respiratória aguda (SDRA) da Europa, EUA, China e Japão.
Alguns dos principais participantes que operam no mercado são a Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Limited., LivaNova PLC, Gilead Sciences, Inc., Fresenius SE & Co. KGaA, Besmed Health Business Corp., Armstrong Medical, Smiths Medical , ResMed, ALung Technologies, Inc., Medtronic, F. Hoffmann-La Roche Ltd, Hamilton Medical, Nice Neotech Medical Systems Pvt. Ltd., Pfizer Inc., WEINMANN Emergency Medical Technology GmbH + Co. KG, NIPRO, Terumo Medical Corporation, Getinge AB.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CAUSE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 ETIOLOGY BY GEOGRAPHY
4.3.1 ETIOLOGY IN U.S.
4.3.2 ETIOLOGY IN EUROPE
4.3.3 ETIOLOGY IN CHINA
4.3.4 ETIOLOGY IN JAPAN
4.4 ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) HEALTHCARE COST PER PATIENT BY GEOGRAPHY
4.5 INSURANCE REIMBURSEMENT
4.5.1 CENTER FOR MEDICARE SERVICES (CMS)–ELSO (EXTRACORPOREAL LIFE SUPPORT ORGANIZATION)
4.5.2 HEALTH RESOURCES AND SERVICES ADMINISTRATION
4.5.3 ABBOTT CODING GUIDE FOR ECMO
4.5.4 CENTRAL GOVERNMENT HEALTH SCHEME (CGHS)
4.5.5 CERN HEALTH INSURANCE SCHEME
4.5.6 AMERICAN SOCIETY OF CLINICAL ONCOLOGY (ASCO) – (MEDICARE & MEDICAID)
4.5.7 AMERICAN HOSPITAL ASSOCIATION
4.5.8 CONCLUSION
4.6 PIPELINE ANALYSIS
5 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: REGULATIONS
5.1 REGULATION IN U.S.:
5.2 LABELING OF MODIFIED DEVICES
5.3 REGULATION IN EUROPE:
5.4 REGULATION IN CHINA:
5.5 REGULATION IN JAPAN:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND INCIDENCE OF ACUTE LUNG INJURY
6.1.2 WIDE RANGE OF RISK FACTORS FOR ACUTE RESPIRATORY DISTRESS SYNDROME
6.1.3 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS
6.1.4 RISING RATE OF AIR POLLUTION AND LIFESTYLE-RELATED DISEASES
6.1.5 INCREASING ACCIDENT RATES AND TRAUMA-CAUSING ARDS
6.2 RESTRAINTS
6.2.1 COMPLICATIONS ASSOCIATED WITH TREATMENTS
6.2.2 HIGH COST OF DEVICE AND TREATMENTS
6.2.3 LACK OF SKILLED WORKFORCE
6.3 OPPORTUNITIES
6.3.1 GROWING GERIATRIC POPULATION
6.3.2 RISING HEALTHCARE EXPENDITURE
6.3.3 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.3.4 INCREASING AWARENESS REGARDING ACUTE RESPIRATORY DISTRESS SYNDROME(ARDS)
6.4 CHALLENGES
6.4.1 STRINGENT RULES & REGULATIONS
6.4.2 MULTIPLE CHALLENGES FACED BY ICU NURSES
7 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE
7.1 OVERVIEW
7.2 CORONAVIRUS DISEASE 2019 (COVID-19)
7.3 SEPSIS
7.4 INHALATION OF HARMFUL SUBSTANCES
7.5 SEVERE PNEUMONIA
7.6 OTHERS
8 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE
8.1 OVERVIEW
8.2 DIAGNOSIS
8.2.1 IMAGING TESTS
8.2.1.1 CHEST X-RAY
8.2.1.2 CT SCAN
8.2.1.3 ULTRASOUND
8.2.1.4 OTHERS
8.2.2 BLOOD TEST
8.2.3 RESPIRATORY RATE
8.2.4 SPO2 TEST
8.2.5 OTHERS
8.3 TREATMENT
8.3.1 MECHANICAL VENTILATION
8.3.1.1 HIGH-FLOW NASAL O2
8.3.1.2 BI-LEVEL POSITIVE AIRWAY PRESSURE
8.3.1.3 CONTINUOUS POSITIVE AIRWAY PRESSURE
8.3.1.4 PRONE POSITIVE VENTILATION
8.3.1.5 OTHERS
8.3.2 CORTICOSTEROIDS
8.3.2.1 METHYLPREDNISOLONE
8.3.2.2 DEXAMETHASONE
8.3.2.3 OTHERS
8.3.3 ANTIVIRAL MEDICATION
8.3.3.1 REMDESIVIR
8.3.3.2 COMBINATION DRUGS
8.3.3.3 OTHERS
8.3.4 EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
8.3.5 TOCILIZUMAB
8.3.6 OTHERS
9 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION
9.1 OVERVIEW
9.2 PARENTERAL
9.2.1 INTRAVENOUS
9.2.2 INTRAMUSCULAR
9.3 ORAL
9.4 OTHERS
10 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.3 SPECIALTY CLINICS
10.4 HOME HEALTHCARE
10.5 OTHERS
11 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 HOSPITAL PHARMACY
11.4 RETAIL PHARMACY
11.5 ONLINE PHARMACY
12 EUROPE
12.1 GERMANY
12.2 FRANCE
12.3 U.K.
12.4 ITALY
12.5 SPAIN
12.6 TURKEY
12.7 HUNGARY
12.8 NETHERLANDS
12.9 SWITZERLAND
12.1 AUSTRIA
12.11 LITHUANIA
12.12 POLAND
12.13 RUSSIA
12.14 IRELAND
12.15 NORWAY
12.16 REST OF EUROPE
13 EUROPE, US, CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: U.S.
13.2 COMPANY SHARE ANALYSIS: EUROPE
13.3 COMPANY SHARE ANALYSIS: JAPAN
13.4 COMPANY SHARE ANALYSIS: CHINA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 GILEAD SCIENCES INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUS ANALYSIS
15.1.3 PRODUCT PORTFOLIO
15.1.4 RECENT DEVELOPMENT
15.2 TERUMO CORPORATION
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUS ANALYSIS
15.2.3 PRODUCT PORTFOLIO
15.2.4 RECENT DEVELOPMENT
15.3 GETINGE AB
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUS ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 LIVANOVA PLC
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 PRODUCT PORTFOLIO
15.4.4 RECENT DEVELOPMENTS
15.5 MEDTRONIC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENTS
15.6 ALUNG TECHNOLOGIES, INC
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 ARMSTRONG MEDICAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 BESMED HEALTH BUSINESS CORP.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 DRÄGERWERK AG & CO. KGAA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 EUROSETS
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 F. HOFFMANN-LA ROCHE LTD
15.11.1 COMPANY SNAPSHOT
15.11.2 RECENT ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.12 FISHER & PAYKEL HEALTHCARE LIMITED
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENTS
15.13 FRESENIUS SE & CO. KGAA
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUS ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 HAMILTON MEDICAL
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 NICE NEOTECH MEDICAL SYSTEMS PVT.LTD.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 NIPRO
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUS ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.17 PFIZER INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUS ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 RESMED
15.18.1 COMPANY SNAPSHOT
15.18.2 REVENUE ANALYSIS
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENT
15.19 SMITHS MEDICAL
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUS ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 WEINMANN EMERGENCY MEDICAL TECHNOLOGY GMBH + CO. KG
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Lista de Tabela
TABLE 1 HEALTHCARE COST FOR ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) ON THE BASIS OF SEVERITY BY COUNTRY IS GIVEN BELOW IN USD:
TABLE 2 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: PIPELINE ANALYSIS
TABLE 3 REGULATION FOR VENTILATORS AND RESPIRATORY DEVICES AS PER FDA
TABLE 4 REGULATION FOR THE USE OF VENTILATOR AND ANESTHESIA GAS MACHINE BREATHING CIRCUIT DEVICES
TABLE 5 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 7 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 8 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 9 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 11 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 12 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 13 EUROPE DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 14 U.S. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 15 CHINA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 16 JAPAN DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 17 EUROPE IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 18 U.S. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 19 CHINA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 20 JAPAN IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 21 EUROPE TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030(USD MILLION)
TABLE 22 U.S. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 23 CHINA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 24 JAPAN TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 25 EUROPE MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 26 U.S. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 27 CHINA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 JAPAN MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 29 EUROPE CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 30 U.S. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 31 CHINA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 JAPAN CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 33 EUROPE ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 34 U.S. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 35 CHINA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 36 JAPAN ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 37 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 38 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 39 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 40 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 41 EUROPE PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 42 U.S. PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 43 CHINA PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 44 JAPAN PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 45 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 47 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 48 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 49 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 50 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 51 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 52 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 53 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 54 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 55 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 56 GERMANY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 57 GERMANY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 58 GERMANY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 59 GERMANY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 60 GERMANY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 61 GERMANY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 62 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 63 GERMANY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 64 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 65 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 66 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 67 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 68 FRANCE DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 69 FRANCE IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 FRANCE TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 71 FRANCE ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 72 FRANCE CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 73 FRANCE MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 74 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 75 FRANCE PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 76 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 78 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 79 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 80 U.K. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.K. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.K. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 83 U.K. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 U.K. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 U.K. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 86 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 87 U.K. PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 88 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 89 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 90 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 91 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 92 ITALY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 93 ITALY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 94 ITALY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 95 ITALY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 96 ITALY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 97 ITALY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 98 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 99 ITALY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 100 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 101 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 102 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 103 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 104 SPAIN DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 105 SPAIN IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 106 SPAIN TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 107 SPAIN ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 108 SPAIN CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 109 SPAIN MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 110 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 111 SPAIN PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 112 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 115 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 TURKEY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 117 TURKEY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 118 TURKEY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 TURKEY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 120 TURKEY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 121 TURKEY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 122 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 123 TURKEY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION
TABLE 124 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 125 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 126 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 127 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 128 HUNGARY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 129 HUNGARY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 130 HUNGARY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 131 HUNGARY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 132 HUNGARY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 133 HUNGARY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 135 HUNGARY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 136 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 137 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 138 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 139 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 140 NETHERLANDS DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 141 NETHERLANDS IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 142 NETHERLANDS TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 143 NETHERLANDS ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 144 NETHERLANDS CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 145 NETHERLANDS MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 146 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 147 NETHERLANDS PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 148 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 149 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 150 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 151 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 152 SWITZERLAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 153 SWITZERLAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 SWITZERLAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 155 SWITZERLAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 156 SWITZERLAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 157 SWITZERLAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 158 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 159 SWITZERLAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 160 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 161 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 162 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 163 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 164 AUSTRIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 165 AUSTRIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 166 AUSTRIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 167 AUSTRIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 168 AUSTRIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 169 AUSTRIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 171 AUSTRIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 172 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 173 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 174 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 175 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 176 LITHUANIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 177 LITHUANIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 178 LITHUANIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 179 LITHUANIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 180 LITHUANIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 181 LITHUANIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 182 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 183 LITHUANIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 184 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 185 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 186 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 187 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 188 POLAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 189 POLAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 POLAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 191 POLAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 192 POLAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 193 POLAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 194 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 195 POLAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 196 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 197 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 198 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 199 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 200 RUSSIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 201 RUSSIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 202 RUSSIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 203 RUSSIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 RUSSIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 205 RUSSIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 206 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 207 RUSSIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 208 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 209 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 210 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 211 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 212 IRELAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 213 IRELAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 214 IRELAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 215 IRELAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 216 IRELAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 217 IRELAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 218 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 219 IRELAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 220 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 221 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 222 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 223 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 224 NORWAY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 225 NORWAY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 226 NORWAY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 227 NORWAY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 228 NORWAY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 229 NORWAY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 230 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 231 NORWAY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 232 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 233 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 234 REST OF EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
Lista de Figura
FIGURE 1 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 2 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: END USER COVERAGE GRID
FIGURE 9 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 11 ACCELERATION IN THE PATIENT POOL OF COVID-19 WITH ARDS IS EXPECTED TO DRIVE THE EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 13 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE U.S.ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 14 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 15 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 16 MOST COMMON PRIMARY CAUSES OF DEATH IN ARDS PATIENTS IN U.S. COUNTRY
FIGURE 17 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
FIGURE 18 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 19 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 20 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 21 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 22 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 23 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 24 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 25 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 26 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 27 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 28 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 29 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 30 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 31 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 32 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 33 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 34 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 35 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 36 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 37 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 38 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 39 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 40 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 41 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 42 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 43 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 44 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 45 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 46 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 47 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 48 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 49 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 50 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 51 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 52 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 53 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 54 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 55 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 56 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 57 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 58 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 59 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 60 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 61 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 62 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 63 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 64 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 65 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 66 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 67 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 68 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 69 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 70 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 71 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 72 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 73 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 74 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 75 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 76 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 77 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 78 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 79 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 80 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 81 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 82 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 83 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 84 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 85 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 86 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 87 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 88 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 89 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 90 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 91 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 92 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 93 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 94 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 95 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 96 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 97 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 98 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SNAPSHOT (2022)
FIGURE 99 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2022)
FIGURE 100 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2023 & 2030)
FIGURE 101 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2022 & 2030)
FIGURE 102 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: CAUSE (2023-2030)
FIGURE 103 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 104 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 105 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 106 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.