Europe Sarcopenia Treatment Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
789.43 Million
USD
1,131.28 Million
2024
2032
USD
789.43 Million
USD
1,131.28 Million
2024
2032
| 2025 –2032 | |
| USD 789.43 Million | |
| USD 1,131.28 Million | |
|
|
|
|
Segmentação do mercado de tratamento de sarcopenia na Europa, por tipo de tratamento (medicamentos, suplementos vitamínicos/dietéticos e outros), tipo (sarcopenia primária e sarcopenia secundária), estágios (pré-sarcopenia, sarcopenia e sarcopenia grave), via de administração (oral, injetável e outros), gênero (masculino e feminino), usuário final (hospitais, clínicas especializadas, assistência médica domiciliar e outros), canal de distribuição (licitação direta, vendas no varejo e outros) - tendências do setor e previsão até 2032
Tamanho do mercado de tratamento de sarcopenia na Europa
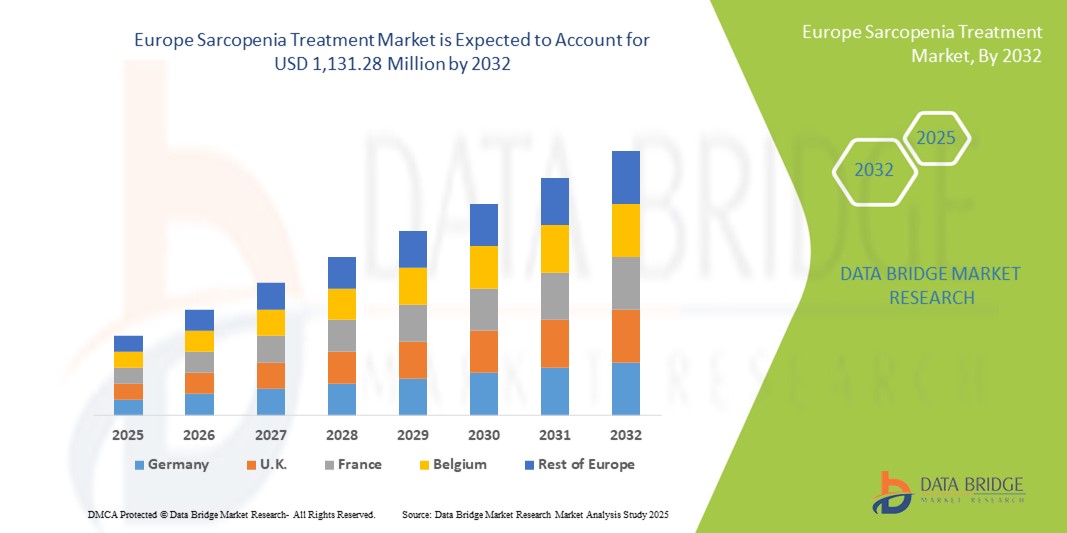
- O tamanho do mercado de tratamento da sarcopenia na Europa foi avaliado em US$ 789,43 milhões em 2024 e deve atingir US$ 1.131,28 milhões até 2032 , com um CAGR de 4,6% durante o período previsto.
- O crescimento do mercado é amplamente impulsionado pela crescente prevalência de desnutrição, deficiências de vitaminas e perda muscular relacionada à idade, juntamente com a crescente conscientização sobre o impacto da sarcopenia na saúde e na qualidade de vida.
- Além disso, a crescente demanda por opções de tratamento eficazes, como suplementação nutricional, fisioterapia e medicamentos, está consolidando os tratamentos para sarcopenia como essenciais para o envelhecimento da população. Esses fatores convergentes estão acelerando a adoção de soluções terapêuticas, impulsionando significativamente o crescimento do setor.
Análise do Mercado de Tratamento de Sarcopenia na Europa
- Os tratamentos para sarcopenia, que incluem suplementos nutricionais, fisioterapia e intervenções farmacológicas, estão se tornando cada vez mais vitais para o controle da perda muscular relacionada à idade e para a manutenção da independência funcional entre a população idosa na Europa, devido à sua eficácia na melhoria da massa muscular, da força e da qualidade de vida em geral.
- A crescente procura por tratamentos para sarcopenia é impulsionada principalmente pelo envelhecimento da população europeia, pela crescente conscientização sobre os impactos da sarcopenia na saúde e pela crescente ênfase em cuidados preventivos e programas de envelhecimento saudável.
- A Alemanha dominou o mercado de tratamento de sarcopenia com a maior participação na receita de 23,9% em 2024, caracterizada por infraestrutura avançada de saúde, altos gastos com saúde e iniciativas governamentais proativas que promovem o cuidado aos idosos, com ampla adoção de programas de suplementação nutricional e fisioterapia.
- Espera-se que a Itália seja o país com crescimento mais rápido no mercado de tratamento de sarcopenia durante o período previsto devido ao aumento dos investimentos em saúde, ao crescimento da população geriátrica e à crescente conscientização sobre a perda muscular relacionada à idade.
- O segmento de suplementos alimentares dominou o mercado de tratamento da sarcopenia com uma participação de mercado de 39,2% em 2024, impulsionado pelo papel bem estabelecido da proteína, vitamina D e cálcio na manutenção e recuperação muscular entre adultos mais velhos
Escopo do Relatório e Segmentação do Mercado de Tratamento de Sarcopenia na Europa
|
Atributos |
Principais insights de mercado sobre o tratamento da sarcopenia na Europa |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Europa
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, análises de preços, análises de participação de marca, pesquisas com consumidores, análises demográficas, análises da cadeia de suprimentos, análises da cadeia de valor, visão geral de matérias-primas/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de tratamento de sarcopenia na Europa
Integração de Saúde Digital e Telemedicina
- Uma tendência significativa e crescente no mercado europeu de tratamento da sarcopenia é a integração de soluções de saúde digital e plataformas de telemedicina, permitindo que pacientes idosos monitorem a saúde muscular, acompanhem a nutrição e recebam orientação remota de profissionais de saúde.
- Por exemplo, o Programa de Telemedicina NutriTrack permite que os pacientes registrem a ingestão alimentar e os exercícios de fortalecimento muscular, enquanto os fisioterapeutas fornecem recomendações em tempo real para ajustes de terapia.
- A integração da saúde digital permite o monitoramento contínuo do progresso do paciente, oferecendo planos de tratamento personalizados e lembretes para suplementação e exercícios, melhorando a adesão e os resultados
- A combinação perfeita de sensores vestíveis , aplicativos móveis e teleconsultas facilita o gerenciamento centralizado do tratamento da sarcopenia, permitindo que os médicos coordenem intervenções nutricionais, terapêuticas e farmacológicas de forma eficiente.
- Esta tendência para o cuidado com a tecnologia está a remodelar as expectativas para a gestão da sarcopenia, com empresas como a PhysioPlus a desenvolver plataformas que integram dados de pacientes, supervisão remota de exercícios e coaching digital.
- A demanda por tratamentos de sarcopenia com suporte de saúde digital está crescendo rapidamente em hospitais, clínicas ambulatoriais e atendimento domiciliar, à medida que os pacientes priorizam cada vez mais a conveniência, o monitoramento e as intervenções personalizadas.
Dinâmica do mercado de tratamento de sarcopenia na Europa
Motorista
Aumento da população geriátrica e conscientização sobre a perda muscular relacionada à idade
- O aumento do envelhecimento da população na Europa, aliado a uma maior consciencialização sobre o impacto da sarcopenia na mobilidade e na qualidade de vida, é um dos principais impulsionadores da procura acrescida de tratamentos eficazes.
- Por exemplo, em 2024, a Alemanha lançou o “Programa de Envelhecimento Saudável”, promovendo a suplementação nutricional e intervenções de exercício em instituições de cuidados a idosos para mitigar a prevalência da sarcopenia.
- À medida que os adultos mais velhos enfrentam maior risco de quedas, fragilidade e declínio funcional, os profissionais de saúde e cuidadores buscam cada vez mais intervenções que melhorem a massa e a força muscular.
- A crescente ênfase nos cuidados de saúde preventivos, incluindo o diagnóstico precoce e o tratamento oportuno da sarcopenia, está a acelerar a adopção de suplementos nutricionais, fisioterapia e opções farmacológicas.
- Campanhas de saúde pública e programas geriátricos estão promovendo a conscientização sobre a sarcopenia, impulsionando a demanda dos pacientes e o envolvimento dos profissionais de saúde na entrega de soluções de tratamento direcionadas.
Restrição/Desafio
Acessibilidade limitada e altos custos de tratamento
- As preocupações em torno da acessibilidade e do preço dos tratamentos para sarcopenia representam um desafio significativo para uma adoção mais ampla pelo mercado em toda a Europa
- Por exemplo, apesar das evidências que apoiam a suplementação de proteínas e a fisioterapia , alguns pacientes idosos na Europa Oriental têm acesso limitado a essas intervenções devido aos altos custos ou à infraestrutura de saúde insuficiente.
- A variabilidade nas políticas de reembolso de assistência médica entre os países pode restringir o acesso dos pacientes a suplementos nutricionais, medicamentos ou sessões especializadas de fisioterapia
- Embora a eficácia do tratamento esteja bem documentada, a falta de diretrizes padronizadas e a conscientização limitada entre os cuidadores podem reduzir a adesão e a aceitação das terapias
- Ensaios clínicos para tratamentos de sarcopenia podem utilizar diferentes critérios diagnósticos, dificultando a comparação dos resultados de diferentes estudos. Essa falta de padronização pode dificultar o desenvolvimento e a aprovação de novos tratamentos por agências reguladoras.
- Superar esses desafios por meio de programas apoiados pelo governo, opções de terapia acessíveis e iniciativas educacionais para pacientes e cuidadores será vital para o crescimento sustentado do mercado.
Escopo do mercado de tratamento de sarcopenia na Europa
O mercado é segmentado com base no tipo de tratamento, tipo de sarcopenia, estágios, via de administração, gênero, usuário final e canal de distribuição.
- Por tipo de tratamento
Com base no tipo de tratamento, o mercado de tratamento da sarcopenia é segmentado em medicamentos, suplementos vitamínicos/dietéticos e outros. O segmento de suplementos vitamínicos/dietéticos dominou o mercado com a maior participação na receita de 39,2% em 2024, impulsionado pelo uso generalizado de suplementação de proteína, vitamina D e cálcio entre a população idosa para prevenir a perda muscular. Os suplementos são preferidos devido à sua segurança, facilidade de administração e capacidade de manter a massa e a função muscular. A crescente conscientização sobre intervenções nutricionais e programas governamentais de apoio impulsiona ainda mais a adoção. Os pacientes frequentemente recebem suplementos como abordagem de primeira linha na sarcopenia primária e secundária. O mercado vê uma demanda estável devido à disponibilidade de alimentos fortificados e fórmulas especializadas com alto teor de proteína. Os profissionais de saúde recomendam suplementos como parte do cuidado preventivo e terapêutico para populações em envelhecimento.
Prevê-se que o segmento de medicamentos apresente a maior taxa de crescimento, de 11,8%, entre 2025 e 2032, impulsionado por novos tratamentos farmacológicos direcionados à atrofia muscular, terapias hormonais e inibidores da miostatina. Medicamentos são cada vez mais prescritos para pacientes com sarcopenia grave ou sarcopenia secundária associada a doenças crônicas. O aumento dos investimentos em P&D e as aprovações regulatórias em países europeus estão acelerando a adoção pelo mercado. Os medicamentos oferecem benefícios direcionados e clinicamente validados para o aumento da massa e força muscular. Campanhas de conscientização estão promovendo seu uso em conjunto com suplementos e fisioterapia. Terapias avançadas oferecem potencial para estratégias de tratamento combinadas, impulsionando ainda mais o crescimento do segmento.
- Por tipo
Com base no tipo, o mercado de tratamento da sarcopenia é segmentado em sarcopenia primária e sarcopenia secundária. O segmento de sarcopenia primária dominou o mercado, com a maior participação na receita, de 55,3% em 2024, impulsionado pela perda muscular relacionada à idade, que afeta a população idosa em toda a Europa. Programas preventivos e de intervenção precoce voltados para o declínio relacionado à idade são comuns em países como Alemanha e França. A sarcopenia primária é frequentemente tratada por meio de intervenções no estilo de vida, suplementação nutricional e fisioterapia. Campanhas de conscientização e programas de saúde geriátrica apoiam a ampla adoção. A prevalência da sarcopenia primária em populações idosas garante uma demanda consistente. Hospitais e clínicas integram medidas preventivas, mantendo o domínio constante do mercado.
Espera-se que o segmento de sarcopenia secundária apresente a taxa de crescimento mais rápida, de 12,3%, entre 2025 e 2032, impulsionado pelo aumento da incidência de sarcopenia devido a condições crônicas como diabetes, DPOC e câncer. O tratamento frequentemente requer uma combinação de medicamentos, suplementação e fisioterapia supervisionada. A crescente pesquisa sobre sarcopenia relacionada a doenças apoia o desenvolvimento de terapias direcionadas. A crescente prevalência de doenças crônicas e relacionadas ao estilo de vida na Europa impulsiona a adoção. Programas clínicos incluem cada vez mais intervenções para sarcopenia secundária. A conscientização entre profissionais de saúde e pacientes contribui para a aceleração da adoção de terapias.
- Por etapas
Com base nos estágios, o mercado de tratamento da sarcopenia é segmentado em pré-sarcopenia, sarcopenia e sarcopenia grave. O estágio de sarcopenia dominou o mercado, com a maior participação na receita, de 47,8% em 2024, visto que os pacientes são tipicamente diagnosticados durante os estágios inicial a moderado. Intervenções como nutrição, exercícios e medicamentos são mais eficazes neste estágio. Programas regulares de triagem e geriatria apoiam a adoção precoce do tratamento. Hospitais e clínicas especializadas enfatizam intervenções oportunas para prevenir a progressão. Campanhas de conscientização e estratégias de gestão proativa contribuem para o domínio do mercado. A adoção é impulsionada pela melhoria dos resultados para os pacientes e pela redução do risco de declínio da mobilidade.
Espera-se que o segmento de sarcopenia grave apresente a taxa de crescimento mais rápida, de 13,1%, entre 2025 e 2032, impulsionado por um número crescente de pacientes idosos com perda muscular avançada. Terapias combinadas intensivas, incluindo medicamentos, suplementação e fisioterapia, são necessárias nesta fase. Os profissionais de saúde se concentram em melhorar a mobilidade, reduzir os riscos de quedas e melhorar a qualidade de vida. O aumento da prevalência de sarcopenia grave entre a população idosa impulsiona a demanda por intervenções eficazes. Programas de gerenciamento de estágio avançado estão sendo implementados em hospitais e clínicas. O aumento da população geriátrica garante um crescimento sustentado do mercado neste segmento.
- Por via de administração
Com base na via de administração, o mercado de tratamento da sarcopenia é segmentado em oral, injetável e outros. O segmento oral dominou o mercado, com a maior participação na receita, de 53,7% em 2024, devido à facilidade de administração de suplementos alimentares e medicamentos por via oral. Os pacientes preferem tratamentos orais por conveniência, adesão ao tratamento e segurança. Suplementos e medicamentos são amplamente disponíveis sem receita médica e recomendados para sarcopenia primária e secundária. Os produtos orais incluem alimentos fortificados, cápsulas e pós adaptados para idosos. A adoção é apoiada por programas de assistência médica domiciliar e recomendações clínicas. O crescimento do mercado é ainda mais impulsionado por campanhas de conscientização pública que promovem a suplementação oral.
Espera-se que o segmento de injetáveis apresente a maior taxa de crescimento, de 14,2%, entre 2025 e 2032, impulsionado pelo desenvolvimento de terapias injetáveis voltadas para sarcopenia grave e tratamentos anabolizantes. Medicamentos injetáveis proporcionam dosagem precisa e eficácia mais rápida em casos avançados. Hospitais e clínicas especializadas estão adotando cada vez mais opções injetáveis para melhorar os resultados dos pacientes. Terapias avançadas permitem a recuperação muscular direcionada em pacientes com sarcopenia crônica ou grave. Ensaios clínicos e aprovações em mercados europeus estão apoiando a adoção. A crescente conscientização sobre a eficácia do tratamento entre profissionais de saúde e pacientes impulsiona o crescimento do segmento.
- Por gênero
Com base no gênero, o mercado de tratamento da sarcopenia é segmentado em masculino e feminino. O segmento feminino dominou o mercado, com a maior participação na receita, de 51,5% em 2024, impulsionado pela maior prevalência de sarcopenia entre mulheres idosas, especialmente na pós-menopausa, devido às alterações hormonais que afetam a massa muscular. Intervenções nutricionais, fisioterapia e medicamentos são comumente recomendados para mulheres. Iniciativas de saúde pública com foco na saúde da mulher apoiam ainda mais a adoção. Campanhas de conscientização visam a detecção precoce e o tratamento em mulheres. Os profissionais de saúde priorizam intervenções personalizadas para mulheres mais velhas. A combinação de cuidados preventivos e tratamentos terapêuticos garante uma demanda sustentada.
Espera-se que o segmento masculino apresente a taxa de crescimento mais rápida, de 10,9%, entre 2025 e 2032, impulsionada pela crescente conscientização sobre a sarcopenia em homens idosos e pela crescente participação em programas de cuidados preventivos. Intervenções no estilo de vida, suplementação e terapias clínicas estão ganhando força entre os homens. Programas governamentais e privados promovem a detecção precoce em homens. O foco crescente em mobilidade, força e independência funcional impulsiona a adoção. Pesquisas clínicas que destacam a sarcopenia em homens sustentam o crescimento. O aumento da população masculina geriátrica garante a expansão do mercado neste segmento.
- Por usuário final
Com base no usuário final, o mercado de tratamento da sarcopenia é segmentado em hospitais, clínicas especializadas, assistência domiciliar e outros. Os hospitais dominaram o mercado, com a maior participação na receita, de 46,2% em 2024, oferecendo tratamento abrangente para sarcopenia, incluindo diagnóstico, suplementação, medicamentos e programas de fisioterapia. Os hospitais estabeleceram programas de cuidados geriátricos, facilitando a adoção do tratamento. A detecção precoce e o monitoramento contínuo em hospitais garantem resultados ideais para os pacientes. Os hospitais frequentemente colaboram com serviços de assistência domiciliar e clínicas para ampliar o atendimento. Hospitais públicos e privados promovem ativamente iniciativas de cuidados preventivos. A abordagem centralizada de tratamento em hospitais garante uma demanda de mercado consistente.
Espera-se que o segmento de assistência médica domiciliar apresente a maior taxa de crescimento, de 15,3%, entre 2025 e 2032, impulsionado pela crescente demanda por cuidados domiciliares para idosos e por intervenções nutricionais e fisioterapêuticas domiciliares. Programas de monitoramento remoto e telemedicina permitem um gerenciamento eficaz fora do ambiente hospitalar. Programas personalizados de assistência domiciliar melhoram a adesão e os resultados do tratamento. O crescimento é sustentado pela crescente conscientização sobre conveniência e independência entre pacientes idosos. Os provedores de assistência médica domiciliar integram ferramentas digitais para monitoramento em tempo real. O segmento se beneficia de políticas de saúde que promovem estratégias de envelhecimento no local.
- Por canal de distribuição
Com base no canal de distribuição, o mercado de tratamento da sarcopenia é segmentado em licitação direta, vendas no varejo e outros. O segmento de vendas no varejo dominou o mercado, com a maior participação na receita, de 49,6% em 2024, impulsionado pela ampla disponibilidade de suplementos e medicamentos em farmácias, supermercados e plataformas de e-commerce. Fácil acessibilidade, disponibilidade de medicamentos sem receita e conveniência sustentam o domínio do mercado. Os consumidores preferem o varejo para compras frequentes e acesso imediato. A expansão do varejo em áreas urbanas e semiurbanas contribui para o crescimento da receita. Campanhas de marketing de marcas direcionadas a cuidadores e idosos aumentam a conscientização. O segmento se beneficia de redes de distribuição estabelecidas e da visibilidade da marca.
Espera-se que o segmento de licitação direta apresente a maior taxa de crescimento, de 12,7%, entre 2025 e 2032, impulsionado pelo aumento da aquisição de produtos para o tratamento da sarcopenia por hospitais, clínicas especializadas e programas governamentais de atendimento a idosos. Pedidos em grandes quantidades em licitações garantem o fornecimento consistente para uso institucional. A crescente conscientização sobre cuidados preventivos e programas voltados para o envelhecimento apoia a distribuição por meio de licitações. Iniciativas governamentais de saúde dependem cada vez mais do fornecimento direto de suplementos e medicamentos. A adoção institucional garante vendas em maior volume e receita sustentada. Parcerias com fabricantes e distribuidores aceleram o crescimento do mercado neste canal.
Análise regional do mercado europeu de tratamento de sarcopenia
- A Alemanha dominou o mercado de tratamento de sarcopenia com a maior participação na receita de 23,9% em 2024, caracterizada por infraestrutura avançada de saúde, altos gastos com saúde e iniciativas governamentais proativas que promovem o cuidado aos idosos, com ampla adoção de programas de suplementação nutricional e fisioterapia.
- Pacientes e profissionais de saúde no país adotam cada vez mais suplementos nutricionais, fisioterapia e medicamentos para intervenção precoce e tratamento da sarcopenia
- A ampla adoção é ainda apoiada por programas governamentais proativos, campanhas de saúde pública e sistemas de cuidados geriátricos bem estabelecidos, tornando os tratamentos de sarcopenia um componente crítico dos cuidados de saúde para idosos.
Visão do mercado de tratamento de sarcopenia na Alemanha
O mercado alemão de tratamento da sarcopenia deteve a maior fatia da receita na Europa em 2024, impulsionado por instalações de saúde avançadas e programas de cuidados geriátricos bem estabelecidos. Os profissionais de saúde enfatizam a detecção precoce e o manejo da sarcopenia por meio de suplementação alimentar, fisioterapia e medicamentos. Campanhas de saúde pública e iniciativas governamentais de cuidado a idosos promovem a conscientização e a adoção de tratamentos. O forte foco da Alemanha em cuidados de saúde preventivos, combinado com altos gastos com saúde, incentiva a integração de abordagens multimodais de manejo da sarcopenia. O mercado está testemunhando crescimento em hospitais, clínicas e atendimento domiciliar, refletindo a ampla aceitação de soluções de tratamento. Os pacientes se beneficiam de opções terapêuticas acessíveis e integração tecnológica no monitoramento e no cuidado.
Visão do mercado de tratamento de sarcopenia na França
O mercado francês de tratamento da sarcopenia deverá expandir-se a uma CAGR significativa durante o período previsto, impulsionado principalmente pelo envelhecimento da população do país e pela crescente conscientização sobre práticas de envelhecimento saudável. Os profissionais de saúde franceses estão cada vez mais recomendando suplementos nutricionais e fisioterapia para prevenir e controlar a sarcopenia. Iniciativas governamentais e programas de saúde preventiva voltados para a população idosa impulsionam ainda mais o crescimento do mercado. A integração de plataformas de telemedicina e saúde digital aumenta a adesão do paciente e o monitoramento do tratamento. Os segmentos de saúde residencial, ambulatorial e domiciliar estão testemunhando uma adoção crescente. A ênfase na qualidade de vida e na independência funcional entre idosos é um impulsionador fundamental para o mercado na França.
Visão do mercado de tratamento de sarcopenia na Itália
Espera-se que o mercado italiano de tratamento da sarcopenia cresça a um CAGR notável, impulsionado pelo aumento da população geriátrica e pela crescente conscientização sobre os riscos à saúde relacionados à perda muscular. Os profissionais de saúde italianos promovem a intervenção precoce por meio de suplementação, programas de exercícios e medicamentos. Iniciativas apoiadas pelo governo e campanhas de saúde preventiva aumentam a adoção, especialmente em ambientes de saúde comunitários e domiciliares. Os pacientes estão cada vez mais engajados em programas de autocuidado, apoiados por saúde digital e telemonitoramento. Hospitais e clínicas especializadas continuam a expandir a oferta de serviços para abordar a sarcopenia de forma abrangente. O crescimento do mercado é impulsionado pelo efeito combinado da ênfase em cuidados preventivos, acessibilidade à saúde e conscientização do paciente.
Visão geral do mercado de tratamento de sarcopenia no Reino Unido
Prevê-se que o mercado de tratamento da sarcopenia no Reino Unido cresça a um CAGR considerável durante o período previsto, impulsionado pela crescente conscientização sobre a deterioração muscular relacionada à idade e programas de saúde preventiva. Os profissionais de saúde estão recomendando suplementação nutricional, fisioterapia e medicamentos para intervenção precoce. Iniciativas governamentais que promovem a saúde do idoso e práticas de envelhecimento saudável apoiam o aumento da adoção. A integração digital da saúde, incluindo monitoramento remoto e telemedicina, aumenta o engajamento do paciente e a adesão ao tratamento. A adoção residencial e clínica de programas de tratamento da sarcopenia está em constante expansão. De modo geral, a ênfase do Reino Unido em cuidados preventivos, independência funcional e intervenções centradas no paciente está impulsionando o crescimento do mercado.
Participação no mercado de tratamento de sarcopenia na Europa
O setor de tratamento da sarcopenia na Europa é liderado principalmente por empresas bem estabelecidas, incluindo:
- Biophytis (França)
- Novartis AG (Suíça)
- Sanofi (França)
- Bayer AG (Alemanha)
- UCB SA (Bélgica)
- Teva Pharmaceutical Industries Ltd. (Israel)
- H. Lundbeck A/S (Dinamarca)
- Galápagos NV (Bélgica)
- Ipsen SA (França)
- Almirall SA (Espanha)
- Orion Corporation (Finlândia)
- Biocryst Pharmaceuticals, Inc. (EUA)
- Haplogen Pharmaceuticals AG (Suíça)
- Medtronic (Irlanda)
- AbbVie Inc. (EUA)
- Pfizer Inc. (EUA)
- Lilly USA, LLC (EUA)
- Bristol-Myers Squibb Company (EUA)
- AstraZeneca (Reino Unido)
Quais são os desenvolvimentos recentes no mercado europeu de tratamento de sarcopenia?
- Em setembro de 2025, a Biophytis revelou sua estratégia de ensaio clínico de Fase 2 para Sarconeos (BIO101), visando a sarcopenia relacionada à obesidade. O ensaio será conduzido na Europa e no Brasil, com o objetivo de avaliar a eficácia do BIO101 na melhora da força e função muscular em pacientes obesos. Essa expansão para a sarcopenia relacionada à obesidade ressalta a versatilidade do BIO101 como um tratamento potencial para múltiplas condições relacionadas à sarcopenia.
- Em agosto de 2025, a Biophytis anunciou ter recebido a aprovação da Agência Europeia de Medicamentos (EMA) e das autoridades reguladoras belgas para iniciar a Parte I do seu ensaio clínico de Fase 3 para o Sarconeos (BIO101), um medicamento candidato para sarcopenia. Esta aprovação marca um passo significativo no desenvolvimento de tratamentos farmacológicos para sarcopenia na Europa.
- Em junho de 2025, a Biophytis anunciou o início do ensaio clínico SARA de Fase 3 para Sarconeos (BIO101), um candidato a medicamento destinado ao tratamento da sarcopenia em idosos. Este ensaio clínico crucial está sendo conduzido em vários países europeus e foi desenvolvido para avaliar a eficácia e a segurança do Sarconeos na melhoria do desempenho físico e da força muscular em pacientes sarcopênicos. O resultado deste ensaio pode abrir caminho para o primeiro tratamento farmacológico para sarcopenia na Europa.
- Em abril de 2025, uma iniciativa europeia liderada pelo grupo COMET (Core Outcome Measures in Effectiveness Trials) desenvolveu um Conjunto de Resultados Essenciais (COS) para sarcopenia. Este COS visa padronizar os resultados medidos em ensaios clínicos e na prática clínica de rotina, garantindo consistência e comparabilidade entre os estudos. A criação deste COS é um passo significativo para melhorar a qualidade das evidências e das estratégias de tratamento para sarcopenia na Europa.
- Em agosto de 2024, a TNF Pharmaceuticals anunciou planos para lançar um ensaio clínico de Fase 2b sobre a eficácia da isomiosamina na sarcopenia e fragilidade no início do primeiro trimestre de 2025. Este ensaio visa explorar ainda mais a eficácia do medicamento na sarcopenia/fragilidade, com base em resultados positivos estatisticamente significativos de um estudo clínico de Fase 2 anterior.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.