Europe Rapid Diagnostic Tests Rdt Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
13.03 Billion
USD
24.49 Billion
2024
2032
USD
13.03 Billion
USD
24.49 Billion
2024
2032
| 2025 –2032 | |
| USD 13.03 Billion | |
| USD 24.49 Billion | |
|
|
|
|
Segmentação do mercado de teste de diagnóstico rápido (RDT) na Europa, por tipo de produto (consumíveis e kits, instrumentos e outros), modo (profissional e sem receita [OTC]), tecnologia (baseada em PCR, ensaios de fluxo contínuo, ensaios imunocromatográficos de fluxo lateral, ensaio de aglutinação, microfluídica , tecnologia de substrato e outros), modalidade (teste baseado em laboratório e teste não baseado em laboratório), faixa etária (adulto e pediátrico), tipo de teste (determinação de confirmação, teste sorológico e sequenciamento viral), abordagem (diagnóstico in vitro e diagnóstico molecular ), espécime (cotonete, sangue, urina, saliva, escarro e outros), aplicação (teste de doenças infecciosas, monitoramento de glicose, teste de cardiologia, teste de oncologia, teste cardiometabólico, teste de drogas de abuso, teste de gravidez e fertilidade, toxicologia) Testes e outros), Usuário final (hospital e clínica, laboratório de diagnóstico, ambiente de atendimento domiciliar, institutos de pesquisa e acadêmicos e outros), canal de distribuição (licitação direta e vendas no varejo) - Tendências do setor e previsão até 2032
Tamanho do mercado de testes de diagnóstico rápido (RDT) na Europa
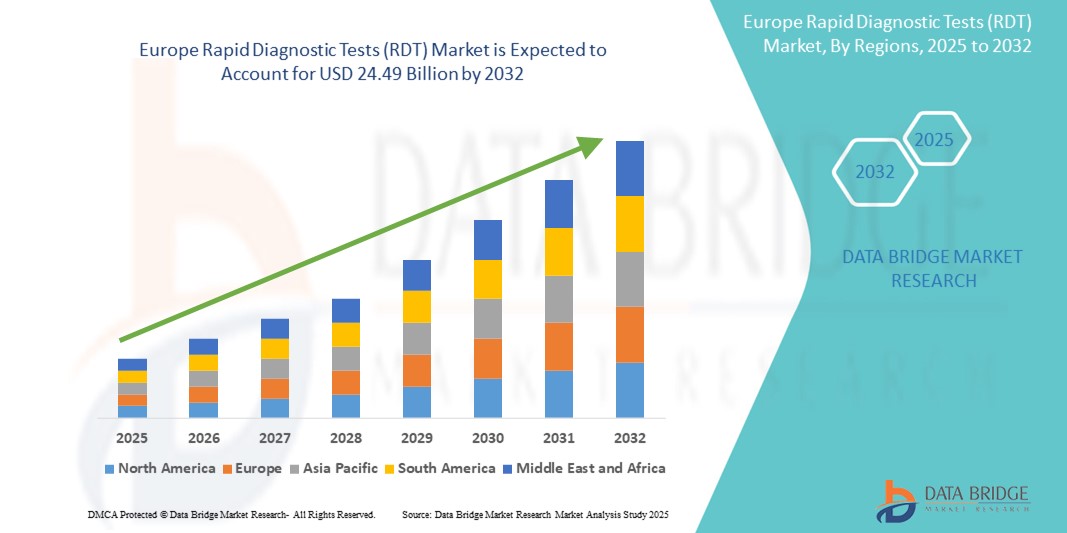
- O tamanho do mercado de testes de diagnóstico rápido (RDT) na Europa foi avaliado em US$ 13,03 bilhões em 2024 e deve atingir US$ 24,49 bilhões até 2032 , com um CAGR de 8,20% durante o período previsto.
- O crescimento do mercado é amplamente impulsionado pela ampla adoção de testes no local de atendimento e em domicílio, possibilitada por rápidos avanços em tecnologias de diagnóstico, como ensaios de fluxo lateral, microfluídica e RDTs moleculares. Isso permite uma detecção mais rápida de doenças e ajuda a reduzir a sobrecarga da infraestrutura laboratorial convencional em ambientes urbanos e rurais na Europa.
- Além disso, a crescente demanda dos consumidores por soluções de diagnóstico rápidas, precisas e acessíveis — especialmente para doenças infecciosas, condições crônicas e triagem de resistência antimicrobiana — está consolidando os RDTs como ferramentas essenciais para intervenção precoce, expansão da telessaúde e vigilância em saúde pública. Esses fatores convergentes estão acelerando a adoção de soluções de diagnóstico rápido, impulsionando significativamente o crescimento do mercado europeu de RDTs.
Análise de mercado de testes de diagnóstico rápido (RDT) na Europa
- Os Testes de Diagnóstico Rápido (RDTs), que oferecem resultados rápidos no local de atendimento sem a necessidade de uma infraestrutura laboratorial complexa, estão se tornando ferramentas essenciais na vigilância de doenças, resposta a surtos e diagnósticos de rotina em ambientes hospitalares e domiciliares devido à sua velocidade, acessibilidade e facilidade de uso.
- A crescente demanda por RDTs é impulsionada principalmente pela crescente prevalência de doenças infecciosas, pela crescente necessidade de assistência médica descentralizada e pelos avanços nas tecnologias de fluxo lateral e imunoensaio que melhoraram a precisão e a vida útil dos testes.
- O Reino Unido dominou o mercado europeu de testes rápidos de diagnóstico (RDT), com a maior participação na receita, de 29,7% em 2024, apoiado por iniciativas governamentais que promovem o autoteste, uma forte rede de farmácias de varejo e o amplo uso de RDTs em testes de COVID-19, gripe e ISTs. A expansão dos serviços de saúde por meio do comércio eletrônico impulsiona ainda mais o diagnóstico domiciliar no país.
- Espera-se que a Alemanha seja o país com crescimento mais rápido no mercado de testes de diagnóstico rápido (RDT) da Europa durante o período previsto, impulsionado pela crescente incidência de doenças crônicas e infecciosas, pelo aumento dos gastos com saúde e pela forte adoção de tecnologias de diagnóstico inovadoras em ambientes hospitalares e de atendimento ambulatorial.
- O segmento de consumíveis e kits dominou o mercado europeu de testes de diagnóstico rápido (RDT) com a maior participação na receita de 64,3% em 2024, atribuída à alta frequência de testes em ambientes de saúde e à facilidade de disponibilidade
Escopo do Relatório e Segmentação do Mercado de Testes de Diagnóstico Rápido (RDT) na Europa
|
Atributos |
Insights sobre o mercado de testes de diagnóstico rápido (RDT) na Europa |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Europa
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, análises de preços, análises de participação de marca, pesquisas com consumidores, análises demográficas, análises da cadeia de suprimentos, análises da cadeia de valor, visão geral de matérias-primas/consumíveis, critérios de seleção de fornecedores, análise PESTLE, análise de Porter e estrutura regulatória. |
Tendências do mercado de testes de diagnóstico rápido (RDT) na Europa
“ Melhoria na precisão e acessibilidade do diagnóstico ”
- Uma tendência significativa e crescente no mercado europeu de testes de diagnóstico rápido (RDT) é a crescente integração de tecnologias avançadas e modelos de teste descentralizados que visam melhorar a velocidade e a acessibilidade do diagnóstico em ambientes clínicos e não clínicos.
- Por exemplo, em maio de 2023, a Roche anunciou o lançamento europeu do seu sistema Cobas Pulse, um dispositivo portátil que combina ferramentas digitais de saúde com testes rápidos no local de atendimento. Essa inovação auxilia os profissionais de saúde a tomar decisões diagnósticas mais rápidas e precisas, à beira do leito ou em ambientes ambulatoriais.
- As plataformas RDT emergentes agora estão equipadas com recursos que permitem a detecção mais rápida de doenças infecciosas, condições crônicas e biomarcadores, usando formatos compactos e fáceis de usar, que não exigem infraestrutura de laboratório central. Essas ferramentas são especialmente úteis em ambientes de emergência, clínicas rurais e atendimento domiciliar, onde resultados oportunos são cruciais.
- Além disso, o crescimento da capacidade de testes multiplex permite que um único teste identifique múltiplos patógenos ou condições simultaneamente. Por exemplo, ensaios de fluxo lateral multiplex podem rastrear simultaneamente influenza, COVID-19 e VSR usando um único cotonete nasal, economizando tempo e recursos e, ao mesmo tempo, melhorando a triagem de pacientes.
- A integração perfeita dos RDTs aos prontuários médicos eletrônicos (PEPs) e aos sistemas de apoio à decisão clínica está aumentando ainda mais sua utilidade clínica. Os profissionais de saúde agora podem acessar resultados em tempo real e integrá-los diretamente aos fluxos de trabalho de gerenciamento de pacientes, otimizando assim o início do tratamento e o acompanhamento.
- Essa tendência em direção a soluções de diagnóstico mais eficientes, fáceis de usar e interoperáveis está remodelando fundamentalmente as expectativas nos sistemas de saúde. Como resultado, empresas como Abbott, Siemens Healthineers e bioMérieux estão expandindo suas ofertas de RDT para incluir soluções profissionais e de venda livre, voltadas para o monitoramento de doenças crônicas e para esforços de triagem em toda a população.
- A demanda por testes de diagnóstico rápido que forneçam resultados rápidos, precisos e acionáveis está crescendo rapidamente em hospitais, atendimento domiciliar e ambientes médicos descentralizados, à medida que agências de saúde pública e provedores privados enfatizam cada vez mais os cuidados preventivos e a intervenção precoce.
Dinâmica do mercado de testes de diagnóstico rápido (RDT) na Europa
Motorista
“Necessidade crescente devido ao aumento da carga de doenças e à demanda por diagnóstico rápido”
- A crescente prevalência de doenças infeciosas, incluindo gripe, COVID-19, VIH, malária e vírus sincicial respiratório (VSR), é um fator significativo para a crescente procura de testes de diagnóstico rápido (TDR) em toda a Europa.
- Por exemplo, em janeiro de 2024, a Roche Diagnostics lançou um novo teste rápido de antígeno duplo, capaz de detectar os vírus da COVID-19 e da influenza A/B, fornecendo resultados em apenas 15 minutos. Isso se alinha ao crescente foco do mercado no diagnóstico precoce e preciso no local de atendimento, o que ajuda a reduzir a transmissão e a melhorar os resultados dos pacientes.
- Governos e sistemas de saúde da região também estão priorizando diagnósticos descentralizados e comunitários para reduzir a pressão sobre os hospitais e aprimorar a vigilância de doenças. Essa tendência é ainda mais impulsionada por iniciativas do Centro Europeu de Prevenção e Controle de Doenças (ECDC) e de agências de saúde pública.
- Além disso, os avanços tecnológicos em ensaios de fluxo lateral, microfluídica e integração de biossensores melhoraram significativamente a precisão, a velocidade e a usabilidade dos RDTs. Essas inovações estão tornando os RDTs uma opção prática e confiável tanto para profissionais quanto para consumidores.
- A conveniência dos testes em casa, especialmente para doenças crônicas e infecções que exigem monitoramento regular, está acelerando a adoção do RDT. A maior conscientização pública, impulsionada pela pandemia, também normalizou o autoteste, incentivando uma aceitação mais ampla em todas as faixas etárias.
Restrição/Desafio
“ Preocupações com a precisão dos testes e a conformidade regulatória ”
- Apesar dos seus benefícios, as preocupações com a sensibilidade e a especificidade de alguns RDTs continuam a desafiar a expansão do mercado. Resultados falsos negativos ou positivos podem levar a diagnósticos errados ou atrasos no tratamento, reduzindo a confiança entre profissionais de saúde e pacientes.
- Por exemplo, estudos realizados durante o início da pandemia da COVID-19 revelaram que vários testes de antigénio de venda livre demonstraram menor sensibilidade em indivíduos assintomáticos, o que levou ao escrutínio regulamentar e à retirada de produtos do mercado.
- Os padrões rigorosos de aprovação em todos os países europeus e as estruturas regulatórias em evolução sob o Regulamento de Diagnóstico In Vitro (IVDR) da UE representam obstáculos adicionais para os fabricantes que pretendem comercializar novos RDTs
- Além disso, as disparidades nas políticas de reembolso entre países como a Alemanha, Itália e Espanha dificultam a adopção equitativa de RDT, particularmente nos serviços de saúde pública e em contextos de poucos recursos.
- O alto custo dos testes rápidos de última geração — como aqueles que usam plataformas de detecção molecular — também pode limitar a adoção em ambientes com restrições orçamentárias, apesar de oferecer maior sensibilidade.
- Para superar essas restrições, as partes interessadas devem se concentrar na validação clínica, na transparência dos dados de desempenho e no alinhamento com os padrões IVDR. Maiores investimentos em fabricação local, parcerias público-privadas e inovação com boa relação custo-benefício serão essenciais para garantir maior acessibilidade e crescimento sustentável do mercado.
Escopo do mercado de testes de diagnóstico rápido (RDT) na Europa
O mercado é segmentado em tipo de produto, modo, tecnologia, modalidade, faixa etária, tipo de teste, abordagem, espécime, aplicação, usuário final e canal de distribuição.
- Por tipo de produto
Com base no tipo de produto, o mercado europeu de testes rápidos de diagnóstico (RDT) é segmentado em consumíveis e kits, instrumentos e outros. O segmento de consumíveis e kits dominou o mercado, com a maior participação na receita, de 64,3% em 2024, devido à alta frequência de testes em ambientes de saúde e à facilidade de disponibilidade.
Espera-se que o segmento de instrumentos testemunhe o CAGR mais rápido de 10,8% entre 2025 e 2032, impulsionado pela crescente demanda por equipamentos de diagnóstico no local de atendimento em clínicas e ambientes remotos.
- Por Modo
Com base no modelo, o mercado europeu de testes rápidos de diagnóstico (RDT) é segmentado em profissionais e de venda livre (OTC). O segmento profissional detinha a maior participação de mercado, 71,5%, em 2024, devido ao amplo uso em hospitais e laboratórios de diagnóstico.
Espera-se que o segmento de medicamentos de venda livre registre o crescimento mais rápido, com um CAGR de 12,4% entre 2025 e 2032, impulsionado pelas tendências crescentes de autoteste e pela disponibilidade do produto em farmácias de varejo.
- Por Tecnologia
Com base na tecnologia, o mercado europeu de testes de diagnóstico rápido (RDT) é segmentado em ensaios baseados em PCR, ensaios de fluxo contínuo, ensaios imunocromatográficos de fluxo lateral, ensaios de aglutinação, microfluídica, tecnologia de substrato e outros. Os ensaios imunocromatográficos de fluxo lateral detiveram a maior participação, 38,9%, em 2024, devido à sua simplicidade e baixo custo.
Espera-se que o segmento de microfluídica cresça na maior CAGR de 13,1% entre 2025 e 2032, impulsionado pelo aumento das tecnologias de laboratório em chip para testes multiplex rápidos.
- Por Modalidade
Com base na modalidade, o mercado europeu de testes de diagnóstico rápido (RDT) é segmentado em testes laboratoriais e testes não laboratoriais. O segmento de testes não laboratoriais liderou o mercado com uma participação de 58,7% em 2024, devido à facilidade de uso e ao menor tempo de resposta.
Espera-se que os testes laboratoriais apresentem o CAGR mais rápido entre 2025 e 2032, pois esses testes oferecem precisão superior e resultados confiáveis, tornando-os vitais para a detecção precoce de doenças infecciosas e condições crônicas.
- Por faixa etária
Com base na faixa etária, o mercado europeu de testes rápidos de diagnóstico (RDT) é segmentado em adulto e pediátrico. O segmento adulto conquistou a maior fatia, 76,4%, em 2024, impulsionado por uma maior carga de doenças e pela demanda por monitoramento de doenças crônicas.
Espera-se que o segmento pediátrico testemunhe o CAGR mais rápido entre 2025 e 2032, devido ao maior foco na detecção precoce de doenças em crianças.
- Por tipo de teste
Com base no tipo de teste, o mercado europeu de testes de diagnóstico rápido (RDT) é segmentado em testes de confirmação, testes sorológicos e sequenciamento viral. Em 2024, o tipo de teste de confirmação dominou o mercado devido à sua alta precisão, amplo uso na detecção de doenças infecciosas e preferência por diagnósticos confirmatórios, como RT-PCR.
Espera-se que os testes sorológicos apresentem o CAGR mais rápido entre 2025 e 2032, impulsionados pela detecção de doenças infecciosas e pelo monitoramento da resposta imunológica.
- Por Abordagem
Com base na abordagem, o mercado europeu de testes de diagnóstico rápido (RDT) é segmentado em diagnóstico in vitro e diagnóstico molecular. O segmento de diagnóstico in vitro manteve a participação dominante devido à acessibilidade e compatibilidade com diversos tipos de analitos.
Espera-se que o segmento de diagnóstico molecular se expanda com o maior CAGR entre 2025 e 2032, devido à precisão e alta sensibilidade.
- Por espécime
Com base na amostra, o mercado europeu de testes rápidos de diagnóstico (RDT) é segmentado em swab, sangue, urina, saliva, escarro e outros. O segmento de swab deteve a maior participação em 2024 devido ao seu amplo uso no rastreamento de doenças infecciosas.
Espera-se que as amostras de sangue apresentem o CAGR mais rápido entre 2025 e 2032, devido à sua alta precisão diagnóstica e amplo uso na detecção de doenças infecciosas e crônicas.
- Por aplicação
Com base na aplicação, o mercado europeu de testes de diagnóstico rápido (RDT) é segmentado em testes de doenças infecciosas, monitoramento de glicose, testes cardiológicos, testes oncológicos, testes cardiometabólicos, testes de drogas de abuso, testes de gravidez e fertilidade, testes toxicológicos e outros. Os testes de doenças infecciosas dominaram o mercado em 2024, com 42,6% de participação, devido ao aumento da preparação para pandemias e à alta demanda por diagnósticos.
Espera-se que os testes cardiometabólicos apresentem o CAGR mais rápido entre 2025 e 2032, devido à crescente prevalência de doenças cardiovasculares e metabólicas, aliada aos avanços em diagnósticos rápidos baseados em biomarcadores.
- Por usuário final
Com base no usuário final, o mercado europeu de testes rápidos de diagnóstico (RDT) é segmentado em hospitais e clínicas, laboratórios de diagnóstico, ambientes de atendimento domiciliar, institutos acadêmicos e de pesquisa, entre outros. O segmento de hospitais e clínicas detinha a maior participação, de 46,2%, em 2024, devido aos altos volumes de testes e à infraestrutura institucional.
O segmento de atendimento domiciliar deverá crescer na mais rápida CAGR de 11,5% entre 2025 e 2032, impulsionado pelas tendências de monitoramento remoto.
- Por canal de distribuição
Com base no canal de distribuição, o mercado europeu de testes de diagnóstico rápido (RDT) é segmentado em licitação direta e vendas no varejo. A licitação direta foi o principal canal em 2024, especialmente para compras institucionais e contratos governamentais.
Espera-se que as vendas no varejo apresentem o CAGR mais rápido entre 2025 e 2032, devido à disponibilidade de kits de teste OTC em farmácias e plataformas online.
Análise regional do mercado europeu de testes de diagnóstico rápido (RDT)
- A Europa dominou o mercado global de testes de diagnóstico rápido (RDT) com uma participação substancial na receita de 40,01% em 2024, impulsionada pelo aumento da demanda por testes no local de atendimento, aumento da carga de doenças infecciosas e expansão dos investimentos governamentais em infraestrutura de saúde pública.
- A região beneficia de um sistema de saúde robusto, de uma elevada consciencialização entre consumidores e profissionais e de uma infraestrutura de diagnóstico avançada, que apoia a adopção generalizada de RDTs.
- Além disso, as iniciativas do Centro Europeu de Prevenção e Controlo de Doenças (ECDC) e o alinhamento com o Regulamento de Diagnóstico In Vitro (IVDR) da UE estão a fortalecer o panorama do diagnóstico e a garantir a fiabilidade e a segurança dos produtos.
Visão geral do mercado de testes de diagnóstico rápido (RDT) do Reino Unido
O mercado de testes de diagnóstico rápido (RDT) do Reino Unido dominou o mercado europeu, com a maior participação na receita, de 29,7% em 2024, impulsionado pela alta adoção de kits de teste domiciliares e fortes iniciativas de saúde pública. O Serviço Nacional de Saúde (NHS) desempenha um papel fundamental na ampla distribuição e subsídios de RDTs para condições como COVID-19, HIV e gripe. Além disso, a robusta infraestrutura de comércio eletrônico do Reino Unido possibilitou maior acesso a RDTs de venda livre para doenças crônicas e infecciosas. Programas de conscientização e campanhas de triagem liderados pelo governo também continuam a promover a detecção precoce e o tratamento preventivo.
Visão do mercado de testes de diagnóstico rápido (RDT) na Alemanha
O mercado alemão de testes diagnósticos rápidos (RDT) deverá ser o de crescimento mais rápido no mercado europeu, com uma taxa composta de crescimento anual (CAGR) de 10,6% entre 2025 e 2032, impulsionado pela inovação tecnológica, pela crescente demanda por diagnósticos descentralizados e pelo aumento dos investimentos em soluções de saúde digital. A sólida base de fabricação de dispositivos médicos e diagnósticos do país está acelerando a disponibilidade doméstica de RDTs. Além disso, a ênfase da Alemanha em sustentabilidade e diagnósticos baseados em dados apoia a integração de RDTs de última geração em práticas de saúde, especialmente em ambientes ambulatoriais e de atendimento remoto.
Visão do mercado de testes de diagnóstico rápido (RDT) na França
O mercado francês de testes rápidos de diagnóstico (RDT) está em constante crescimento, apoiado por políticas de reembolso favoráveis e parcerias público-privadas crescentes para preparação para pandemias e vigilância de doenças. O país também está testemunhando uma crescente adoção de testes rápidos para a saúde da mulher, marcadores cardiovasculares e condições respiratórias em ambientes urbanos e rurais.
Visão do mercado de testes de diagnóstico rápido (RDT) na Itália
O mercado italiano de testes diagnósticos rápidos (RDT) está em expansão devido ao foco crescente em saúde preventiva e à modernização de sua infraestrutura de diagnóstico pós-COVID-19. A crescente demanda por testes domiciliares e diagnósticos em farmácias no local de atendimento está remodelando a prestação de serviços de saúde tanto no norte quanto no sul da Itália.
Participação no mercado de testes de diagnóstico rápido (RDT) na Europa
O setor de testes de diagnóstico rápido (RDT) é liderado principalmente por empresas bem estabelecidas, incluindo:
- Abbott (EUA)
- Danaher (EUA)
- Cellex (EUA)
- Fujirebio (Japão)
- AdvaCare Pharma (EUA)
- ACESSO BIO (EUA)
- Cardinal Health (EUA)
- Bio-Rad Laboratories, Inc. (EUA)
- Cefeida (EUA)
- BD (EUA)
- F. Hoffmann-La Roche Ltd (Suíça)
- BIOMÉRIEUX (França)
- InBios International, Inc. (EUA)
- Luminex Corporation (EUA)
- Gnomegen LLC (EUA)
- QIAGEN (Holanda)
- Quidel Corporation (EUA)
- Sysmex Corporation (Japão)
- Siemens Healthineers AG (Alemanha)
- MEGACOR Diagnostik GmbH (Áustria)
- PerkinElmer (EUA)
- Sekisui Diagnostics (EUA/Japão)
- Diagnósticos PTS (EUA)
- Werfen (Espanha)
- Nova Biomedical (EUA)
- Trinity Biotech (Irlanda)
Últimos desenvolvimentos no mercado europeu de testes de diagnóstico rápido (RDT)
- Em maio de 2023, a F. HOFFMANN-LA ROCHE LTD anunciou oficialmente a aquisição da Stratos Genomics. Essa aquisição levou ao desenvolvimento do sequenciamento baseado em DNA para uso diagnóstico. Isso fortaleceu o segmento de diagnóstico em saúde da empresa, gerando maior receita para a empresa.
- Em abril de 2023, em julho, o Painel Respiratório BIOFIRE 2.1 plus da bioMérieux SA, que testa 23 patógenos, incluindo a infecção por SARS-CoV-2, responsável por infecções do trato respiratório, está disponível comercialmente para uso em todo o mundo e auxilia no diagnóstico precoce de infecções respiratórias. Este desenvolvimento ajudou a empresa a gerar mais receita.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.